Understanding Product Development Technologies for Medical Devices
Explore the significance and evolution of product development technologies in medical...

In the rapidly evolving landscape of medical technology, prototype services are crucial for transforming innovative concepts into viable products. These services streamline the development process and ensure that medical devices comply with stringent regulatory standards and meet user expectations. As the industry faces growing demands for speed and precision, organizations must leverage effective prototyping to mitigate risks and enhance patient outcomes. A closer examination of prototype services reveals their essential contributions to the success of medical device development.
In medical device development, prototype services involve a series of structured processes and methodologies designed to create preliminary models that closely resemble the final product in both functionality and appearance. These services typically classified as prototype services include key stages such as:
Prototyping is essential for validating concepts, ensuring compliance with stringent regulatory standards, and gathering user feedback, which is crucial for refining the product.
Statistics show that effective prototyping can significantly mitigate the risk of costly redesigns and delays. Rapid prototyping allows manufacturers to transition from digital designs to physical prototypes in just days. This accelerated timeline is vital in a market where speed is not merely an advantage but a necessity for success. For instance, the global healthcare equipment engineering market, valued at USD 8.2 billion in 2023, is projected to expand at a CAGR of 10.1%, underscoring the increasing demand for effective prototyping processes.
Successful case studies, such as the collaboration between Medtronic and the University of Galway, illustrate how dedicated prototype hubs can enhance innovation pathways and foster collaboration within the medtech ecosystem. This partnership aims to expedite the advancement of healthcare technologies, ultimately leading to practical solutions that improve patient outcomes.
Expert opinions highlight the strategic importance of prototyping in healthcare equipment development. Industry leaders assert that prototyping is not merely a technical step but a strategic process that informs every phase of product creation. Prototype services effectively bridge the gap between theoretical ideas and practical application by producing tangible representations of concepts, ensuring that healthcare tools are both innovative and safe for end-users.
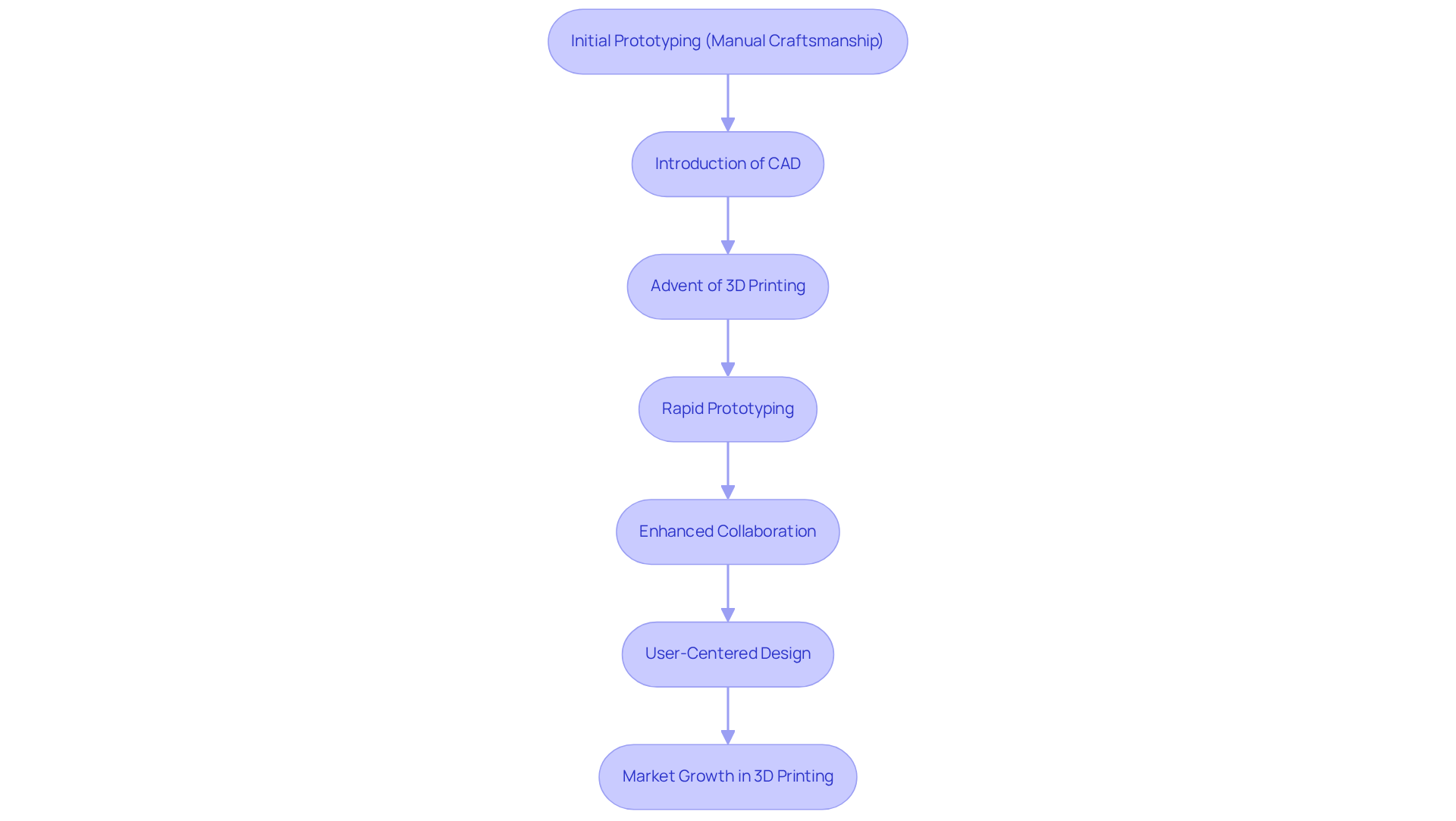
Technological advancements and the evolving needs of the industry have profoundly shaped the evolution of prototype services in the creation of medical devices. Initially, prototyping was limited by manual craftsmanship and basic materials, which constrained both speed and accuracy. However, the advent of computer-aided drafting (CAD) and 3D printing technologies has transformed this landscape. Rapid prototyping now facilitates quicker iterations and more precise models, enabling engineers to assess form, fit, function, and usability early in the development process.
The integration of digital tools has not only accelerated prototyping timelines but also enhanced collaboration among design teams, fostering innovation and compliance in healthcare products. For instance, the 3D printing sector in healthcare is projected to grow by USD 5.74 billion at a compound annual growth rate (CAGR) of 23.31% from 2023 to 2028, driven by the increasing demand for customized healthcare tools tailored to individual patient needs. This trend is reshaping the healthcare sector by enabling the development of patient-specific implants, surgical guides, and complex medical instruments, underscoring Voler Systems' commitment to advancing innovative medical technology.
Moreover, case studies highlight the impact of these technologies: the rise of minimally invasive robotic surgery has led to a significant increase in demand for equipment such as the da Vinci surgical robot, with the market expected to expand from USD 5.5 billion in 2018 to USD 24 billion by 2025. This illustrates how advanced prototyping methods can directly influence market trends and patient outcomes, aligning with Voler Systems' role in compliant and reliable electronic product development.
Currently, prototype services emphasize user-centered design, regulatory compliance, and the utilization of advanced materials that enhance performance. Nevertheless, the substantial initial setup costs for 3D printing facilities present a considerable challenge for new market entrants. As the regulatory landscape for 3D-printed healthcare products continues to evolve, companies like Voler Systems are encouraged to adopt cost-saving strategies and collaborate with industry stakeholders to address challenges effectively. The ongoing advancement of bioprinting technology, which employs living cells to create intricate structures, further exemplifies the potential of CAD and 3D printing in revolutionizing healthcare solutions.
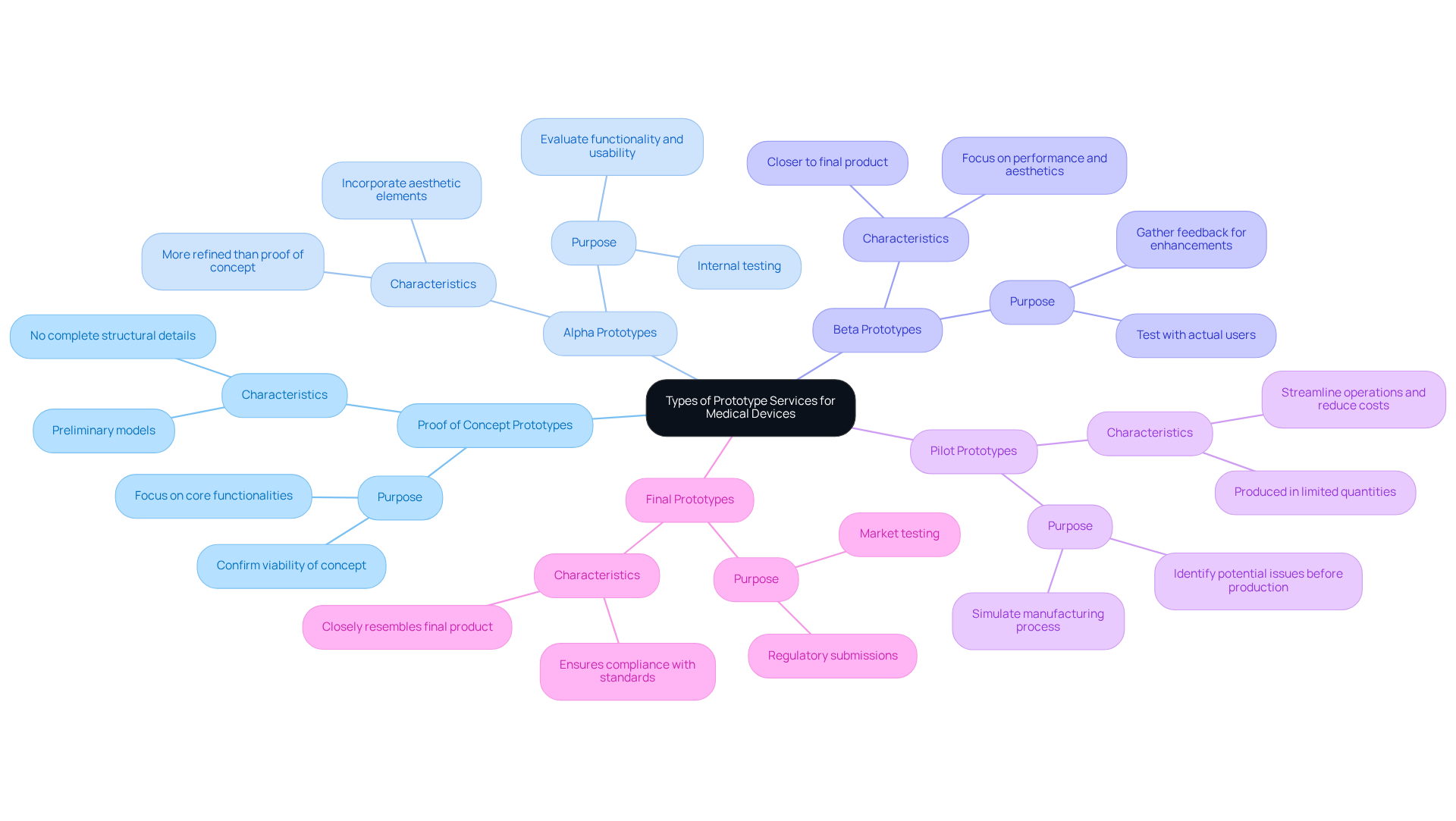
In medical device development, various types of prototype services are utilized, each serving a distinct purpose in the design and validation process:
Proof of Concept Prototypes: These preliminary models are essential for confirming the viability of a concept, concentrating on core functionalities without delving into complete structural details. They play a critical role in determining whether an idea can evolve into a viable product.
Alpha Prototypes: More refined than proof of concept prototypes, alpha prototypes incorporate aesthetic elements and are used for internal testing. This stage evaluates functionality and usability, enabling teams to identify and address issues early in the development cycle.
Beta Prototypes: These prototypes are closer to the final product and are frequently tested with actual users. Feedback gathered during this phase is invaluable for enhancing both performance and aesthetics, ensuring that the product meets user expectations.
Pilot Prototypes: Produced in limited quantities, pilot prototypes simulate the manufacturing process. This stage is crucial for identifying potential issues prior to full-scale production, aiding in the streamlining of operations and cost reduction.
Final Prototypes: The last iteration closely resembles the final product and is utilized for regulatory submissions and market testing. This stage ensures that the equipment complies with all necessary standards and is ready for launch.
Each type of prototype services plays a vital role in the iterative development process, ensuring that the final healthcare product not only meets user requirements but also adheres to stringent regulatory standards. As the sector advances, the integration of cutting-edge technologies, such as AI-driven optimization of layouts, is enhancing the efficiency and effectiveness of these prototyping phases, ultimately leading to faster and more reliable product development.
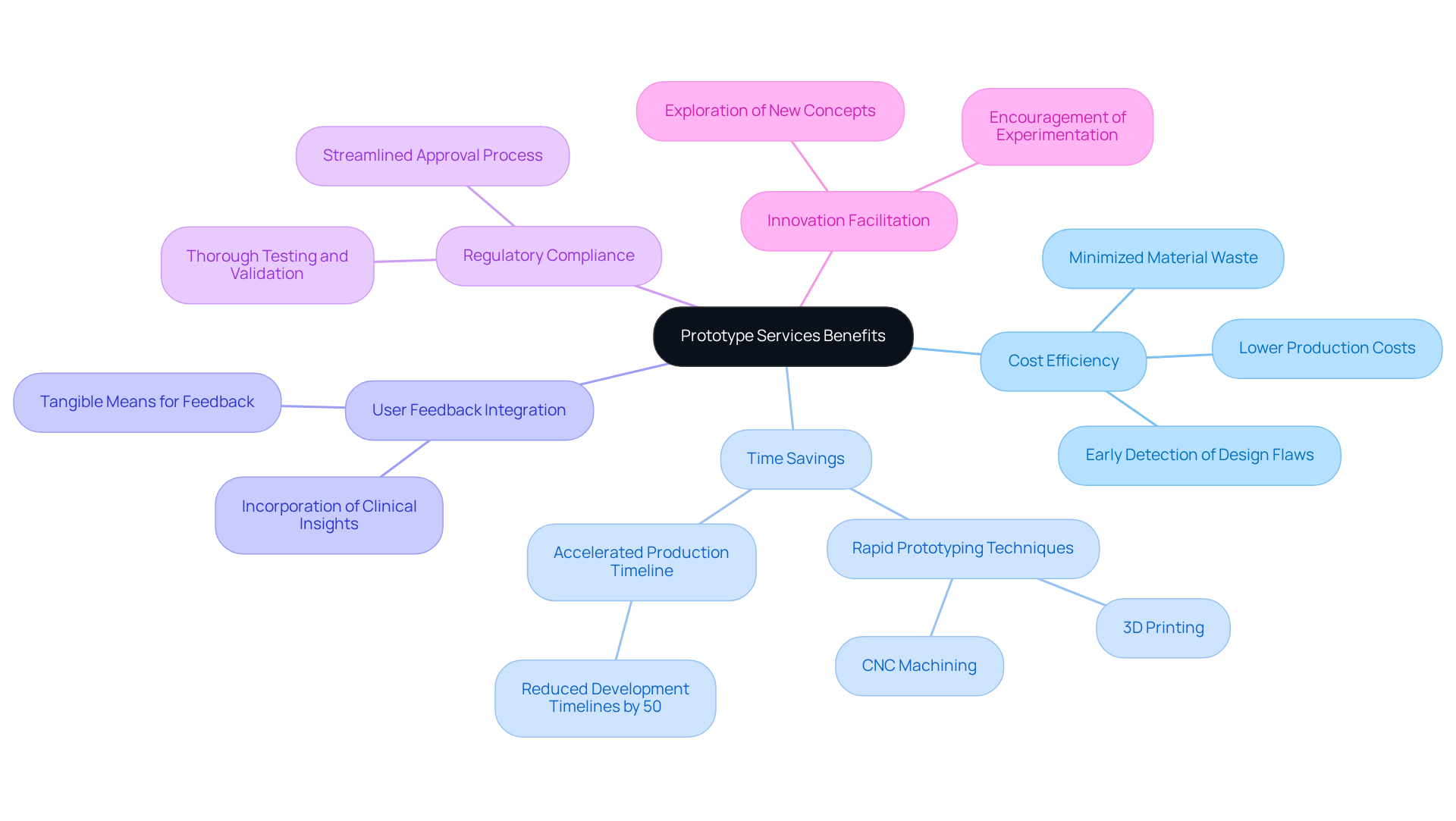
Prototype services offer significant advantages crucial for the successful creation of medical devices. The key characteristics include:
Cost Efficiency: Prototyping facilitates early detection of design flaws, thereby reducing the risk of expensive rework in later development stages. This approach minimizes material waste and lowers overall production costs, making innovation more accessible for both startups and established healthcare organizations.
Time Savings: Rapid prototyping techniques, such as 3D printing and CNC machining, enable quicker iterations, thereby accelerating the overall production timeline and facilitating faster market entry. This acceleration can reduce development timelines by up to 50%, which is vital in the competitive landscape of medical equipment.
User Feedback Integration: Prototypes serve as a tangible means to gather user feedback, ensuring that the final product aligns with the needs and expectations of healthcare professionals and patients. The incorporation of clinical insights, as highlighted in the case study 'Integration with Clinical Feedback,' enhances usability and safety features, ultimately improving the effectiveness of the device.
Regulatory Compliance: Prototyping plays a critical role in ensuring that products meet stringent regulatory standards by allowing for thorough testing and validation prior to submission. Voler Systems' compliance review process ensures that prototypes adhere to emissions and ESD requirements, streamlining the approval process and reducing time to market.
Innovation Facilitation: By fostering experimentation and exploration of new concepts, prototyping encourages innovation in healthcare equipment design. These benefits highlight the crucial role that prototype services have in the development of safe, effective, and market-ready medical devices.
Incorporating testimonials from satisfied clients can further illustrate the effectiveness of Voler Systems' prototype services, enhancing trust and credibility. For a comprehensive understanding of the benefits and processes involved, please refer to the table of contents.
Prototype services in medical device development are essential for creating innovative and effective healthcare solutions. By transforming concepts into tangible prototypes, these services validate ideas, ensure compliance with regulatory standards, and gather vital user feedback. This iterative process significantly mitigates the risks associated with costly redesigns and delays, highlighting the strategic importance of prototyping in the fast-paced medical device industry.
The article emphasizes the structured stages of prototyping, including:
The evolution of these services from manual craftsmanship to advanced technologies such as CAD and 3D printing has transformed the development landscape, enabling quicker iterations and fostering collaboration among design teams. Various types of prototypes - ranging from proof of concept to final prototypes - each play a crucial role in refining products to meet user needs and regulatory requirements.
Ultimately, embracing prototype services is not merely about keeping pace with industry demands; it is about driving innovation and improving patient outcomes. As the healthcare sector continues to evolve, organizations must prioritize effective prototyping strategies to navigate challenges and leverage emerging technologies. By doing so, they can ensure the development of safe, compliant, and market-ready medical devices that genuinely enhance the quality of care.
What are prototype services in medical device development?
Prototype services in medical device development involve structured processes and methodologies to create preliminary models that closely resemble the final product in functionality and appearance.
What key stages are included in prototype services?
Key stages in prototype services include conceptualization, creation, fabrication, and rigorous testing of prototypes.
Why is prototyping essential in medical device development?
Prototyping is essential for validating concepts, ensuring compliance with regulatory standards, and gathering user feedback, which is crucial for refining the product.
How does effective prototyping impact redesigns and delays?
Effective prototyping can significantly mitigate the risk of costly redesigns and delays.
What is rapid prototyping and why is it important?
Rapid prototyping allows manufacturers to transition from digital designs to physical prototypes in just days, which is vital in a market where speed is necessary for success.
What is the projected growth of the global healthcare equipment engineering market?
The global healthcare equipment engineering market, valued at USD 8.2 billion in 2023, is projected to expand at a CAGR of 10.1%.
Can you provide an example of a successful collaboration in prototype services?
An example is the collaboration between Medtronic and the University of Galway, which aims to enhance innovation pathways and expedite the advancement of healthcare technologies.
What do industry experts say about the role of prototyping in healthcare equipment development?
Industry experts assert that prototyping is a strategic process that informs every phase of product creation, bridging the gap between theoretical ideas and practical application.
