10 Essential Elements of Product Design for Medical Devices
Explore the essential elements of product design for effective medical devices and...
Embedded systems are transforming the medical device landscape, acting as the foundation for innovations that improve patient care and safety. As the healthcare sector evolves, it is crucial for engineers and developers to grasp the complexities of these systems to deliver high-quality, compliant products. Given the rapid technological advancements and stringent regulatory requirements, stakeholders must consider how to ensure that their embedded systems not only adhere to clinical standards but also foster innovative solutions in an increasingly competitive market.
Embedded technologies represent advanced computing solutions that integrate hardware and software to perform specific functions within medical devices. These frameworks are crucial for real-time health monitoring, diagnostics, and automated treatment procedures. For example, a pacemaker utilizes an integrated system to continuously monitor heart rhythms and deliver electrical impulses as needed, showcasing the device's life-saving capabilities.
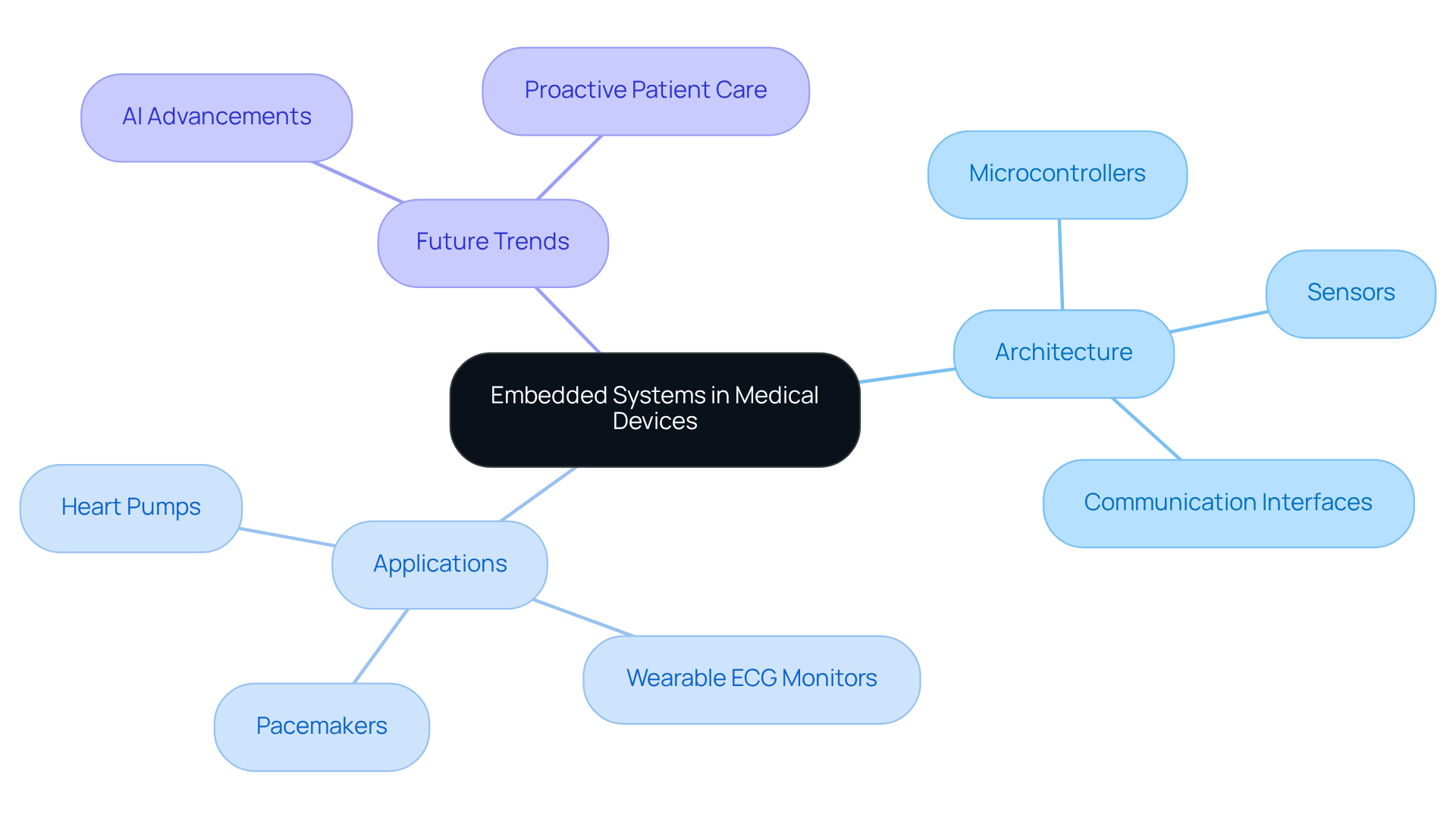
A comprehensive understanding of the architecture of these systems, including microcontrollers, sensors, and communication interfaces, is vital for engineers and developers involved in topics in embedded systems. This expertise ensures that equipment is designed to meet clinical requirements and regulatory standards, while also fostering innovation that can significantly enhance performance and patient outcomes.
As we look ahead to 2026, the importance of embedded technologies in healthcare innovation continues to grow, driven by advancements in AI and data-driven models that enable more proactive patient care. Voler Systems has developed a range of healthcare instruments, such as wearable ECG monitors, heart pumps, and liquid biopsy platforms, which exemplify how integrated technologies can improve diagnostic accuracy and patient safety. As the healthcare landscape evolves, the integration of robust embedded systems will remain fundamental to the effective functionality of medical devices, particularly concerning the topics in embedded systems, ensuring that they operate with precision and compliance in an ever-changing environment.
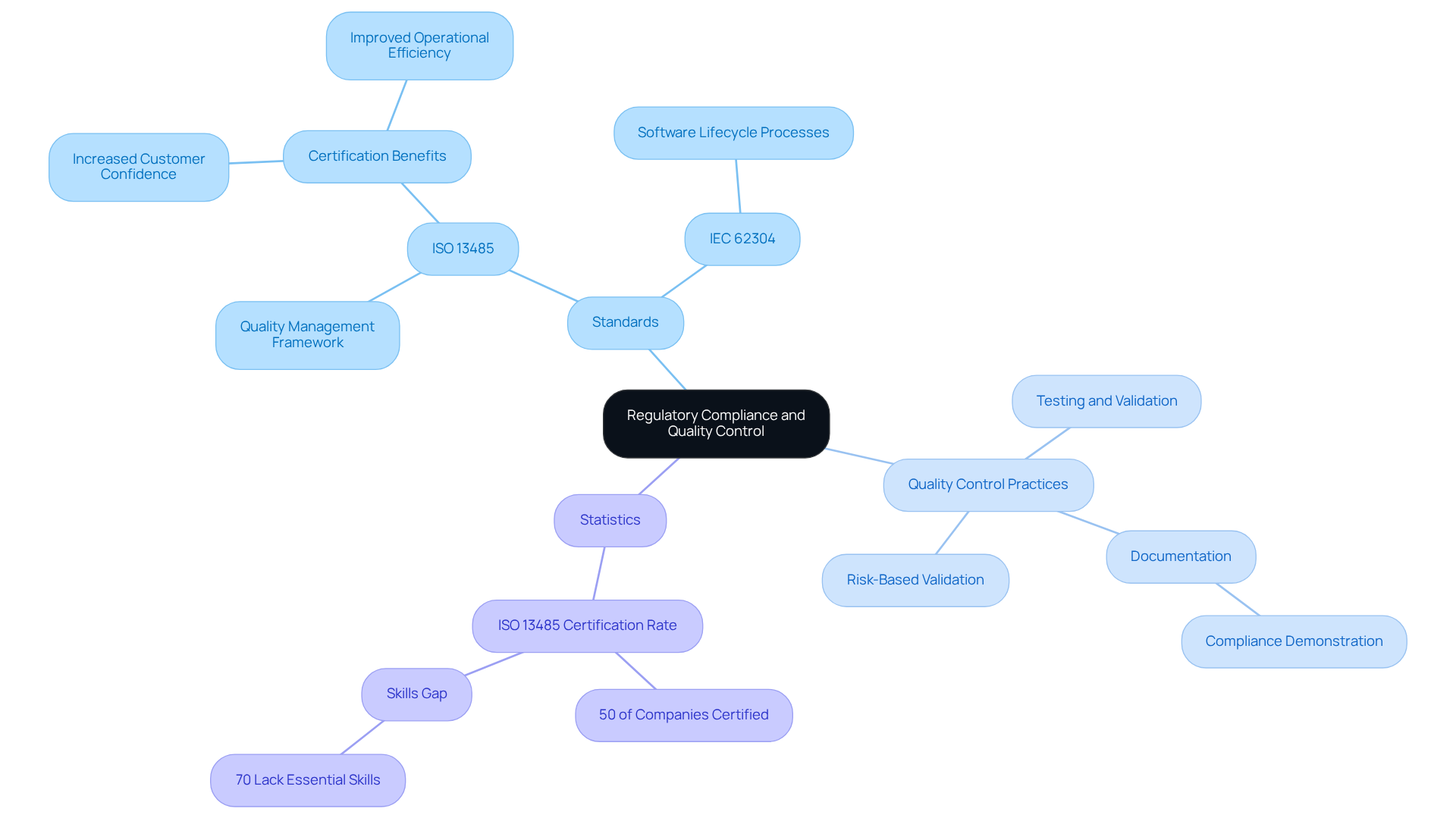
To ensure the safety and efficacy of healthcare products, producers must adhere to strict regulatory criteria established by organizations such as the FDA and ISO. Central to these standards are:
Implementing a comprehensive quality control framework requires regular testing and validation of both hardware and software components. For example, employing risk-based validation strategies is crucial for identifying potential hazards and effectively mitigating them.
Moreover, maintaining meticulous documentation throughout the development process is vital for demonstrating compliance during audits and inspections. Prioritizing regulatory compliance and quality control not only enhances a company's reputation but also significantly reduces the risk of costly recalls and legal challenges. Notably, statistics reveal that while 50% of healthcare equipment companies are ISO 13485 certified, nearly 70% lack the essential skills to meet regulatory requirements efficiently. This gap underscores the critical need for manufacturers to invest in quality management systems to ensure competitiveness and safeguard customer safety in the healthcare equipment sector.
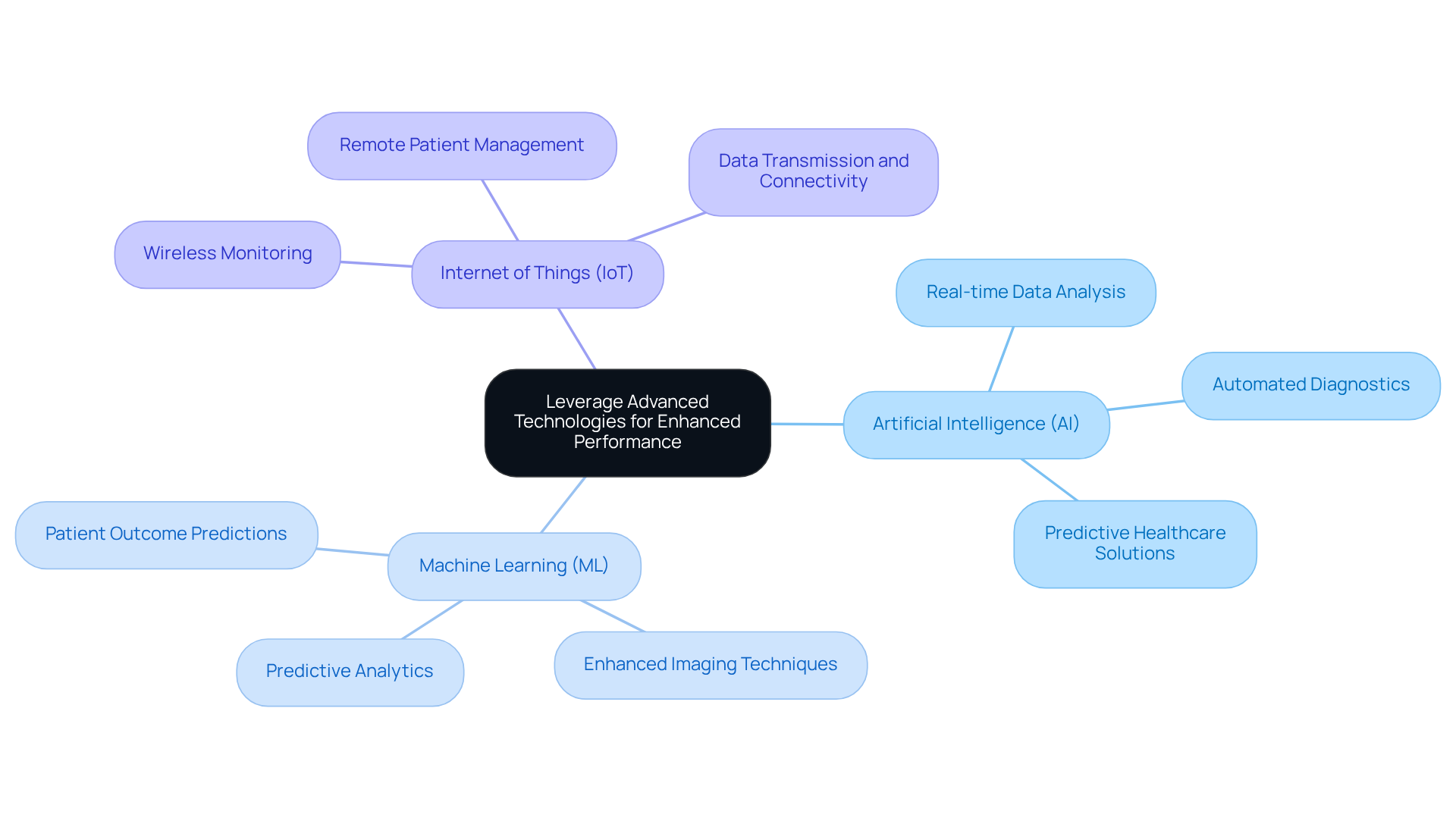
Incorporating advanced innovations such as artificial intelligence (AI), machine learning (ML), and the Internet of Things (IoT) into medical instruments significantly enhances their functionality and efficacy. AI algorithms, for instance, can analyze patient data in real-time, providing actionable insights that enable clinicians to make informed decisions swiftly. Furthermore, IoT connectivity allows systems to transmit data wirelessly, facilitating remote monitoring and timely interventions, which are crucial for improving patient outcomes.
A notable case study on the transition to wireless cardiac monitoring exemplifies the effective application of these technologies in practice. Recent statistics indicate that nearly half of U.S. adults utilize health applications, with a substantial portion relying on wearable technology to monitor health metrics. This trend underscores the growing adoption of IoT in healthcare.
Additionally, the implementation of ultra-low power designs is vital for battery-operated equipment, ensuring long-lasting performance essential for patient adherence. By embracing these technologies, manufacturers can create innovative solutions that not only address current healthcare challenges but also anticipate future needs, positioning themselves at the forefront of medical device innovation.
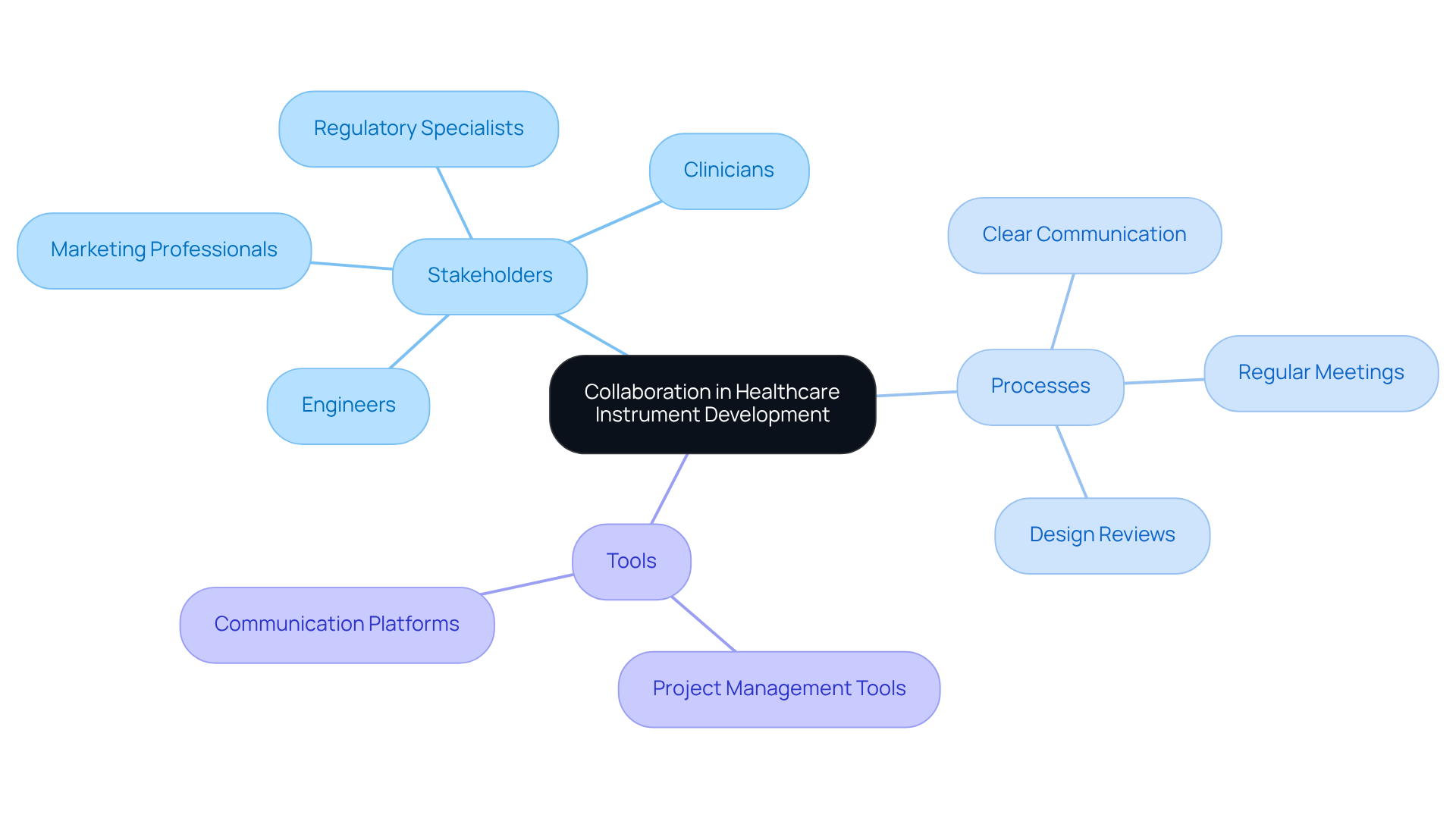
Successful healthcare instrument development hinges on effective collaboration among diverse stakeholders, including engineers, regulatory specialists, clinicians, and marketing professionals. Clear communication channels and regular meetings are vital for facilitating knowledge sharing and aligning project goals. For example, cross-functional teams can conduct thorough design reviews, ensuring that all aspects of the device - from functionality to regulatory compliance - are meticulously addressed.
Voler Systems excels in providing documentation compliance assistance, guiding startups through the complex regulatory landscape of the healthcare equipment sector. Additionally, modern innovations such as project management tools and communication platforms significantly enhance collaboration and streamline workflows. By employing advanced design techniques related to topics in embedded systems, including FPGA and AI technologies, organizations can improve battery life for wireless healthcare equipment, ensuring reliable performance.
Fostering a culture of open feedback empowers team members to voice concerns and propose innovative solutions, creating an environment where creativity flourishes. By prioritizing interdisciplinary collaboration and leveraging technology, organizations can optimize the development process, substantially reduce time-to-market, and ultimately deliver high-quality medical devices that effectively address patient needs.
Embedded systems are crucial in advancing medical devices, seamlessly integrating hardware and software to ensure precise functionality and enhance patient care. For engineers and developers, understanding these systems is vital, as they underpin innovative healthcare solutions that comply with stringent regulatory standards.
This article has explored key topics such as:
The importance of adhering to ISO and FDA standards is highlighted, along with the transformative effects of AI, IoT, and effective teamwork in the development of medical devices. Each of these components plays a role in improving device performance and ensuring patient safety within a rapidly evolving healthcare landscape.
As the medical device industry continues to innovate, adopting these best practices is essential for manufacturers striving to remain competitive and deliver high-quality products. By prioritizing embedded systems, regulatory compliance, advanced technologies, and collaborative efforts, stakeholders can significantly enhance patient outcomes and propel the future of healthcare technology. The call to action is clear: invest in knowledge, foster teamwork, and leverage cutting-edge innovations to shape the next generation of medical devices.
What are embedded systems in medical devices?
Embedded systems in medical devices are advanced computing solutions that combine hardware and software to perform specific functions, essential for real-time health monitoring, diagnostics, and automated treatment procedures.
Can you provide an example of an embedded system in a medical device?
A pacemaker is an example of an embedded system; it continuously monitors heart rhythms and delivers electrical impulses as needed, demonstrating its life-saving capabilities.
Why is understanding the architecture of embedded systems important for engineers and developers?
Understanding the architecture, including microcontrollers, sensors, and communication interfaces, is crucial for ensuring that medical equipment meets clinical requirements and regulatory standards while fostering innovation to enhance performance and patient outcomes.
How is the role of embedded technologies in healthcare expected to change by 2026?
By 2026, the role of embedded technologies in healthcare is expected to grow, driven by advancements in AI and data-driven models that facilitate more proactive patient care.
What types of healthcare instruments has Voler Systems developed?
Voler Systems has developed healthcare instruments such as wearable ECG monitors, heart pumps, and liquid biopsy platforms, which demonstrate how integrated technologies can enhance diagnostic accuracy and patient safety.
Why is the integration of embedded systems important in the evolving healthcare landscape?
The integration of robust embedded systems is fundamental to the effective functionality of medical devices, ensuring they operate with precision and compliance in an ever-changing healthcare environment.
