Understanding the IoT Product Development Process for Medical Devices
Explore the essential stages of the IoT product development process for medical devices.

Creating effective hardware board designs for medical devices presents a complex challenge that merges innovation with stringent regulatory requirements. Engineers and designers must navigate the dual demands of ensuring compliance with evolving standards while integrating advanced technologies to enhance functionality. As the industry prepares for significant regulatory changes in the coming years, a critical question emerges: how can professionals effectively address these challenges to not only achieve compliance but also drive innovation that improves patient outcomes? This article explores best practices that will empower engineers to design safer, more effective medical devices, ensuring they are well-prepared for the future of healthcare technology.
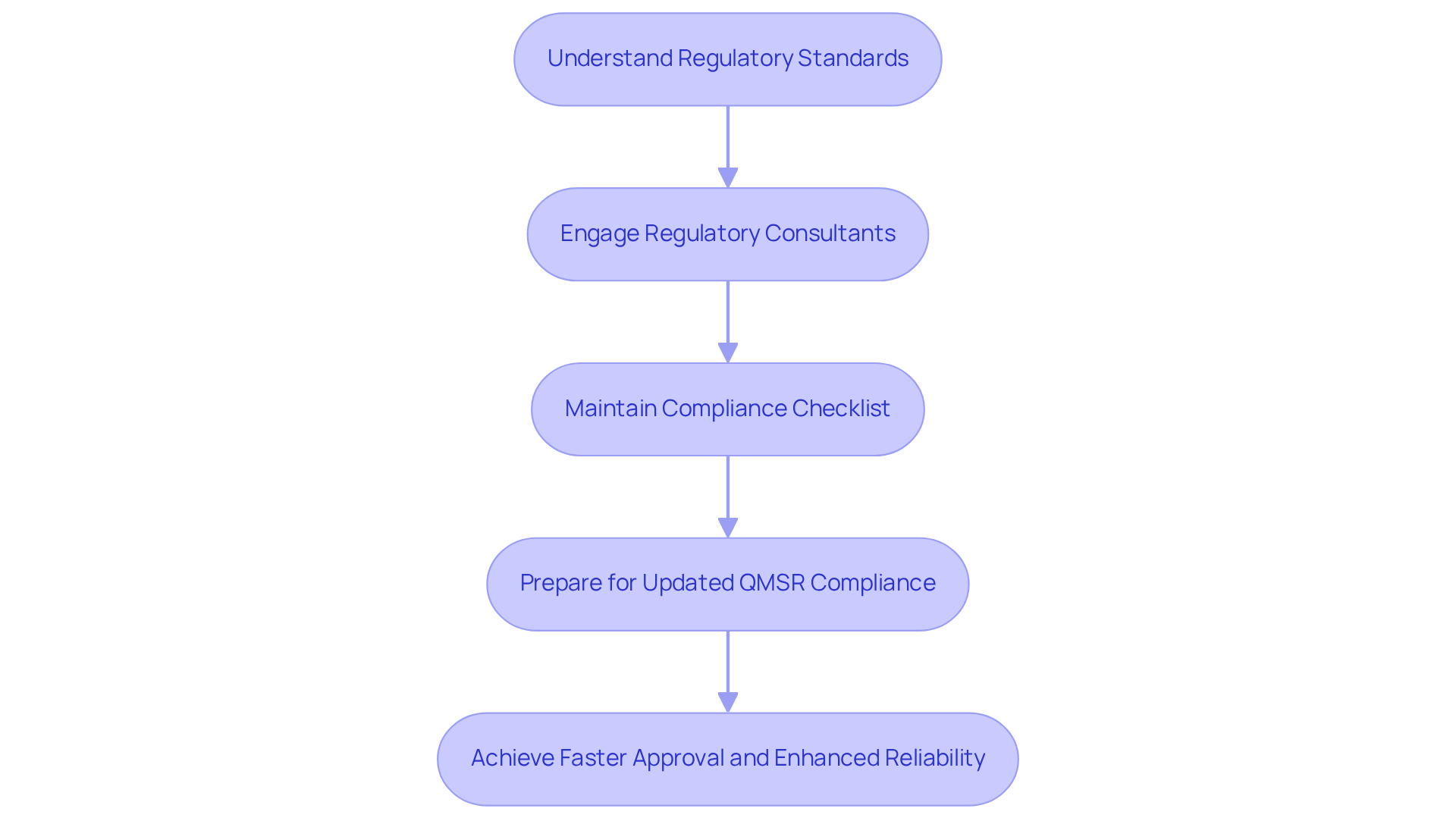
Creating effective hardware board design for medical equipment necessitates a comprehensive understanding of the regulatory standards governing the industry. Adherence to ISO 13485 and FDA regulations is essential to ensure that products are safe and effective for patient use. Engineers must become familiar with the specific requirements relevant to their device category, particularly concerning risk management protocols and documentation practices.
Engaging regulatory consultants early in the planning process can yield valuable insights, facilitating a smoother approval process. Furthermore, maintaining a detailed checklist of compliance requirements throughout the development stages is crucial to avoid costly redesigns and delays.
As of February 2026, compliance with the updated FDA Quality Management System Regulation (QMSR) will be imperative, as submissions must demonstrate conformity to these standards to avert application denials under the FD&C Act. Successful case studies illustrate that proactive compliance not only accelerates approval timelines but also enhances product reliability and market readiness.
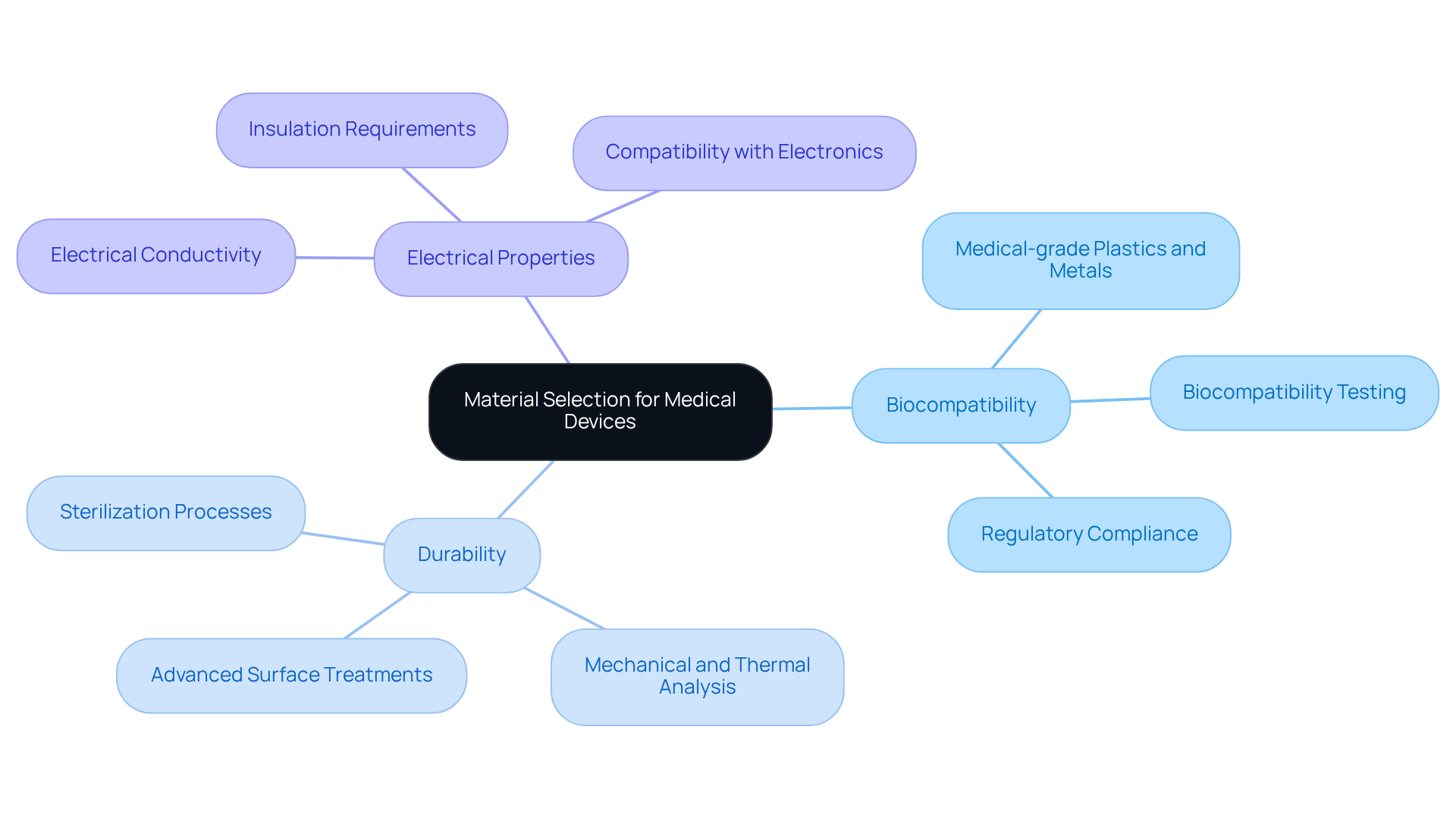
Choosing suitable supplies and components is essential in the hardware board design for medical devices. Key considerations include:
Medical-grade plastics and metals must be selected for their ability to endure sterilization processes and their compatibility with human tissue. Interacting with trustworthy suppliers who provide thorough specifications and compliance documentation is crucial to mitigate risks associated with product failure. Additionally, comprehensive testing - including mechanical and thermal analysis - ensures that the selected materials will function reliably under expected conditions.
Recent advancements in biocompatible substances, such as new polycarbonate copolymers and SILTEM HU resins, underscore the ongoing evolution in science, offering safer alternatives that meet stringent regulatory standards. As highlighted by Prof. Łukasz Szymański, the choice of materials plays a fundamental role in the biocompatibility of healthcare products, emphasizing the necessity for thorough testing to streamline regulatory approval and enhance patient safety.
Furthermore, case studies demonstrate that materials such as LNP ELCRES CRX copolymer resins significantly improve performance, underscoring the importance of informed selections in achieving successful outcomes. Voler Systems' commitment to comprehensive documentation compliance support for startups in healthcare technology further emphasizes the significance of integrating innovative materials into product development, ultimately enhancing performance and patient safety.
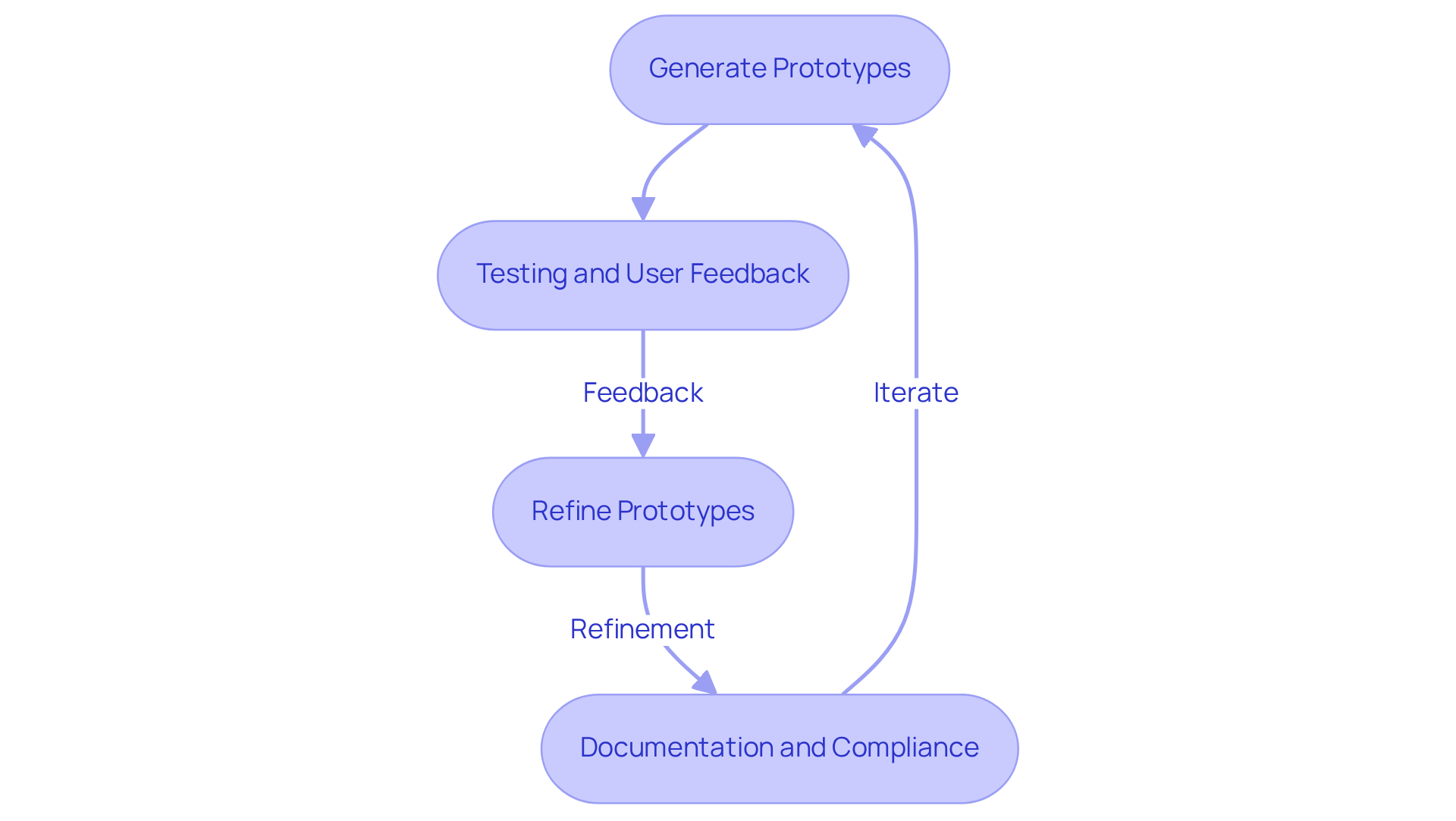
An iterative creation process is essential for the successful development of hardware board design in medical devices. This methodology entails generating multiple prototypes, each refined through rigorous testing and user feedback. Techniques such as rapid prototyping and simulation, along with hardware board design, enable engineers to quickly evaluate viability and implement necessary adjustments. Research indicates that iterative testing can significantly enhance user recognition rates, with advancements in anatomic recognition achieving up to 98% across development iterations.
Regular design reviews and active stakeholder engagement during the prototyping phase promote innovative solutions and improve user experience. Furthermore, meticulous documentation of each iteration not only tracks changes and rationales but also ensures compliance with regulatory standards, serving as a valuable resource for future projects. Voler Systems exemplifies this approach by providing extensive documentation compliance assistance, which includes detailed user manuals and case studies to aid startups in the healthcare sector in navigating regulatory challenges effectively. Their XLerator program condenses weeks of prototyping into a single day, thereby accelerating the development timeline and reducing costs. By adopting these practices, manufacturers of healthcare tools can ensure their products are intuitive, effective, and aligned with clinical needs.
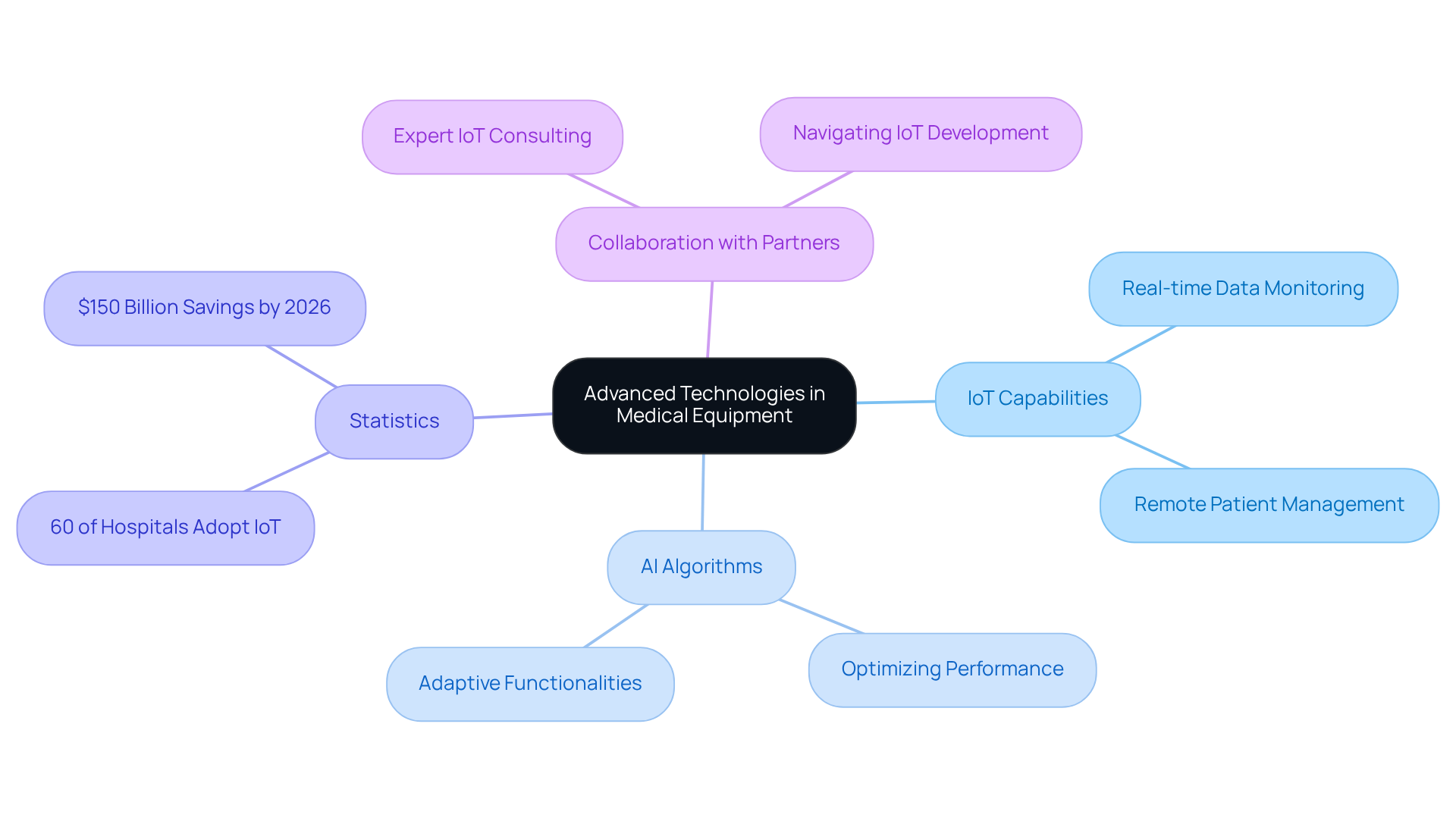
Incorporating advanced technologies into the hardware board design significantly enhances medical equipment capabilities. The integration of IoT capabilities, for example, enables real-time data monitoring and remote patient management, which can lead to improved patient outcomes. Statistics indicate that over 60% of hospitals worldwide have adopted IoT technology for various healthcare applications, underscoring the growing reliance on connected technologies. Additionally, AI algorithms can optimize equipment performance by facilitating adaptive functionalities based on user interactions. The healthcare sector is projected to save up to $150 billion annually by 2026 through enhanced diagnostics and operational efficiencies driven by AI.
Voler Systems provides expert IoT consulting and comprehensive support, assisting manufacturers in navigating the complexities of IoT development to produce high-quality, secure products. Engineers must remain vigilant regarding the latest technological advancements and explore their applications to enhance product design. Collaborating with technology partners like Voler Systems can also grant access to innovative solutions that improve product offerings, ensuring that medical devices not only meet current demands but also adapt to future healthcare challenges.
Creating effective hardware board designs for medical devices requires a multifaceted approach that integrates regulatory compliance, material selection, iterative design, and advanced technology. By prioritizing these elements, engineers can ensure their products are safe, effective, and responsive to the evolving demands of the healthcare industry.
Understanding regulatory standards such as ISO 13485 and FDA regulations is crucial for product approval and patient safety. The selection of appropriate materials, which must be biocompatible and durable, is equally important. Advancements in materials science can significantly enhance device performance. Additionally, the iterative design and prototyping processes are vital, allowing for continuous refinement based on user feedback and rigorous testing. Incorporating advanced technologies like IoT and AI can further enhance the functionality of medical devices, ultimately leading to improved patient outcomes.
In summary, embracing these best practices streamlines the design process and positions medical devices for success in a competitive marketplace. As the healthcare landscape evolves, staying informed about regulatory changes and technological advancements is essential. Manufacturers are encouraged to adopt these strategies to meet current standards and anticipate future challenges, ensuring their products remain at the forefront of innovation and patient care.
Why is understanding regulatory standards important in hardware board design for medical equipment?
Understanding regulatory standards is crucial because it ensures that products are safe and effective for patient use, adhering to guidelines such as ISO 13485 and FDA regulations.
What are the key regulatory standards mentioned for medical equipment?
The key regulatory standards mentioned are ISO 13485 and FDA regulations.
How can engineers ensure compliance with regulatory standards?
Engineers can ensure compliance by familiarizing themselves with specific requirements relevant to their device category, particularly regarding risk management protocols and documentation practices.
What role do regulatory consultants play in the design process?
Engaging regulatory consultants early in the planning process can provide valuable insights and facilitate a smoother approval process.
What is the significance of maintaining a compliance checklist during development?
Maintaining a detailed checklist of compliance requirements is crucial to avoid costly redesigns and delays throughout the development stages.
What is the deadline for compliance with the updated FDA Quality Management System Regulation (QMSR)?
Compliance with the updated FDA Quality Management System Regulation (QMSR) will be imperative as of February 2026.
What are the consequences of failing to demonstrate conformity to FDA standards?
Failing to demonstrate conformity to FDA standards can lead to application denials under the FD&C Act.
How does proactive compliance impact product development?
Proactive compliance can accelerate approval timelines and enhance product reliability and market readiness, as illustrated by successful case studies.
