Master Product Development and Engineering for Medical Devices
Master best practices for product development and engineering in medical devices for...

The rapid evolution of technology has significantly transformed the healthcare landscape, with the Internet of Things (IoT) leading this change. As IoT devices become increasingly integrated into medical settings, it is essential for stakeholders to understand the structured framework of the IoT product development process. This understanding is crucial for enhancing patient care and operational efficiency.
However, navigating the complexities of this development process raises critical questions:
By exploring these elements, we uncover not only the potential for innovation but also the challenges that must be addressed to fully realize the benefits of IoT in healthcare.
The structured framework of the IoT product development process is designed to create, develop, and deploy Internet of Things (IoT) devices, especially in the healthcare sector. The IoT product development process encompasses critical stages, including:
All aimed at ensuring that the final product meets user needs and adheres to industry standards. Given the stringent regulations governing healthcare instruments, the IoT product development process is vital for mitigating risks and achieving successful outcomes.
The integration of IoT technology into healthcare instruments significantly enhances monitoring capabilities, data collection, and overall patient care. By 2026, it is projected that approximately 70% of healthcare instruments will incorporate IoT technology, highlighting the necessity of adhering to a comprehensive IoT product development process. Expert insights indicate that a well-defined approach not only streamlines the development phases but also bolsters the reliability and effectiveness of medical IoT solutions, ultimately leading to improved patient outcomes and satisfaction.
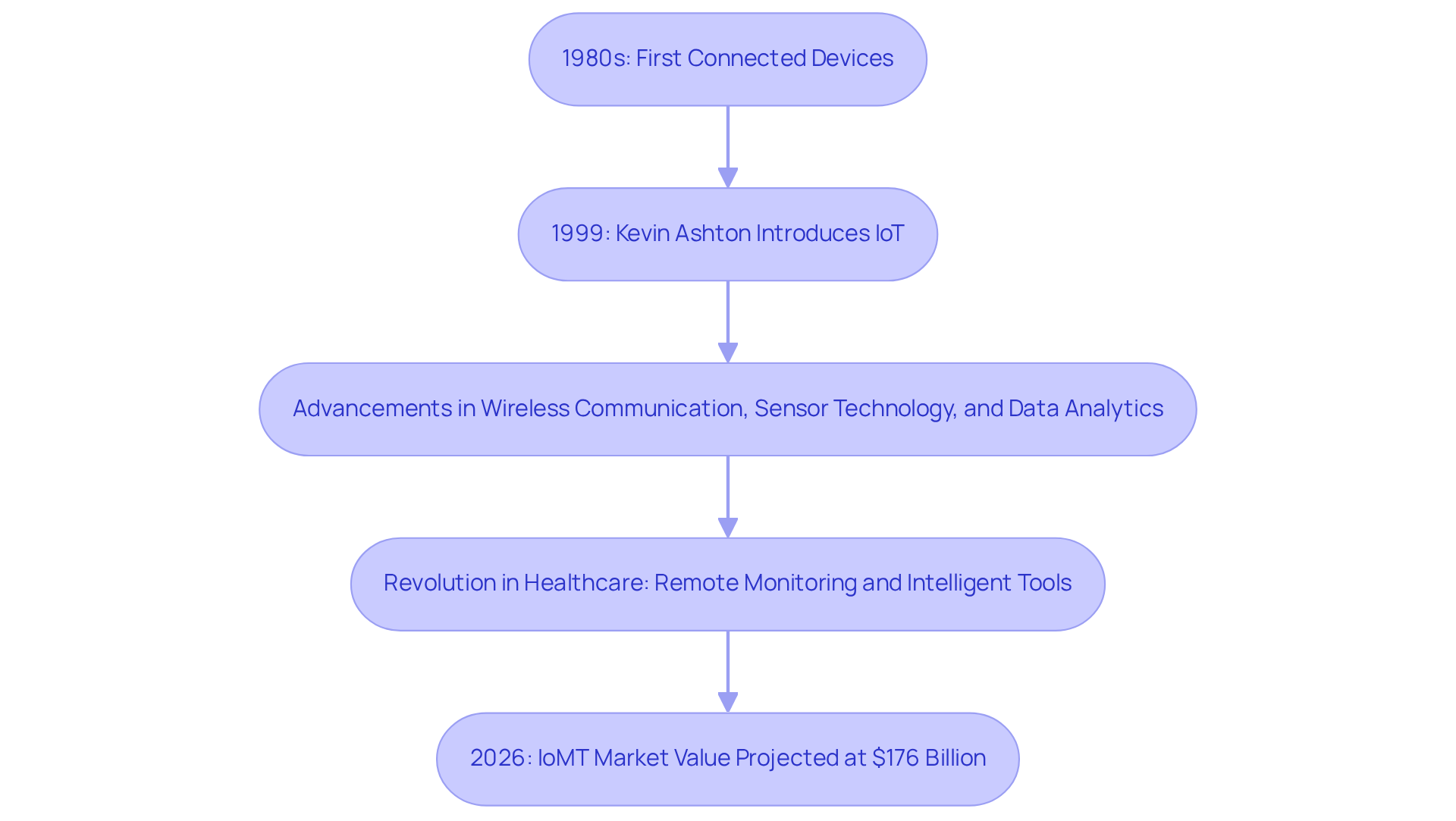
The concept of the Internet of Things (IoT) traces back to the early 1980s, when the first connected devices emerged from academic and industrial research. However, it was in 1999 that Kevin Ashton introduced the term 'Internet of Things,' marking a pivotal moment in the evolution of connected objects. Since that time, advancements in wireless communication, sensor technology, and data analytics have significantly propelled the development of IoT.
In the healthcare sector, the integration of IoT has revolutionized the monitoring of individuals and the delivery of services, leading to innovations such as remote health monitoring systems and intelligent healthcare tools. This progression underscores the increasing recognition of the potential for interconnected devices to enhance patient outcomes and streamline healthcare processes.
Notably, the Internet of Medical Things (IoMT) is anticipated to reach a market value of $176 billion by 2026, reflecting the rising demand for IoT solutions within the healthcare industry.
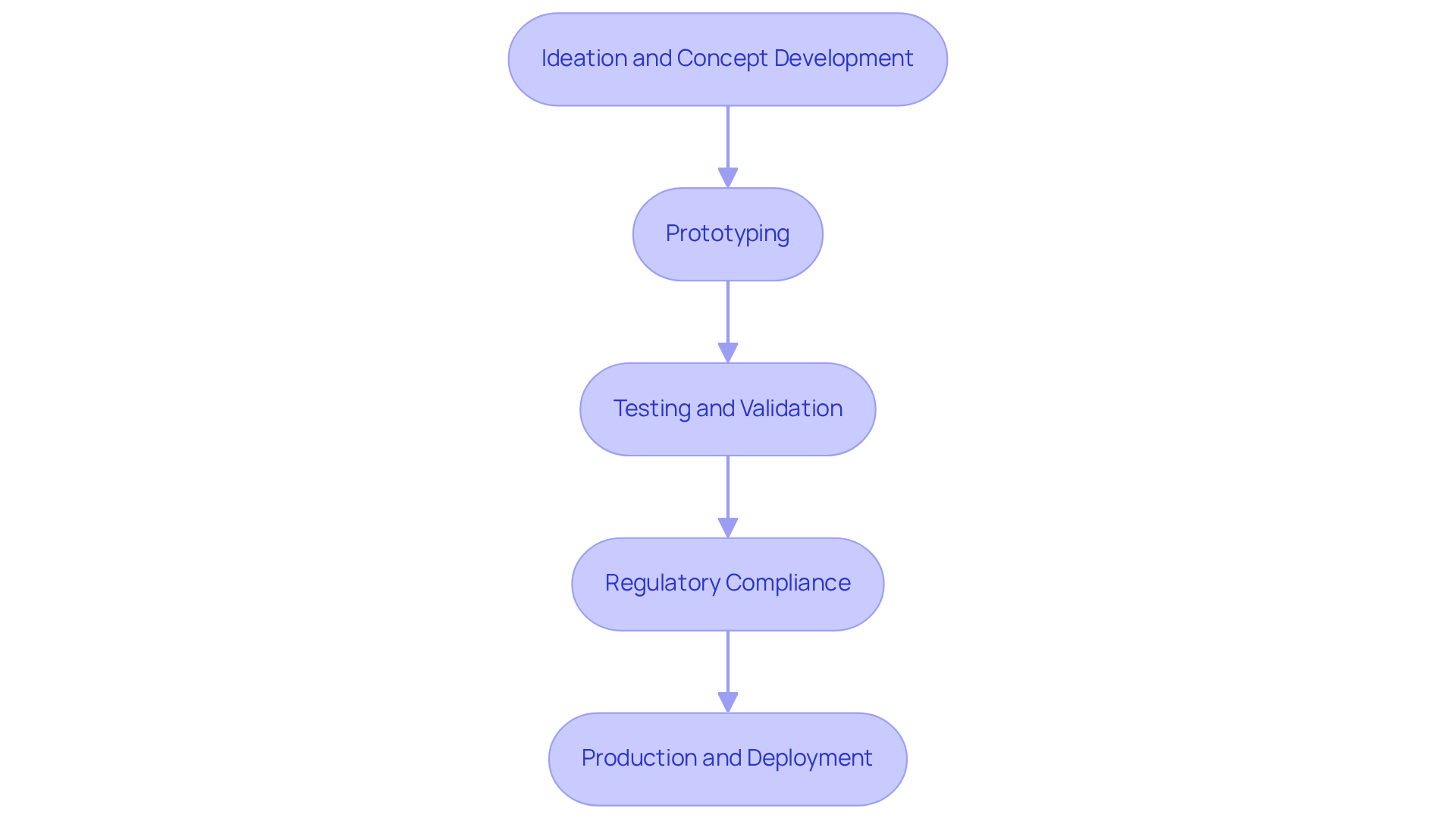
The IoT product development process consists of several essential stages:
Ideation and Concept Development: This initial phase centers on identifying a market need and conceptualizing a viable solution.
Prototyping: The IoT product development process includes developing a working model, which is crucial for testing the feasibility of the concept. Prototyping allows for iterative enhancements driven by user input, which is particularly vital in medical equipment development where accuracy and reliability are paramount. For example, the HemeChip project demonstrated rapid prototyping, enabling the team to gather efficacy data and prepare for clinical trials within a compressed timeline.
Testing and Validation: Rigorous testing is necessary in the IoT product development process to ensure that the equipment meets safety and performance standards. This includes Engineering Validation Testing (EVT), which evaluates functional stability and the integration of hardware and firmware. Challenges during EVT may involve integration issues and data transmission bottlenecks, which must be resolved to ensure a stable design. Recognizing common pitfalls in manufacturing tests is critical for guaranteeing quality and efficiency in electronic product design, and Voler Systems offers expertise in navigating these challenges.
Regulatory Compliance: Navigating the complex landscape of healthcare equipment regulations is essential for the IoT product development process to obtain necessary approvals. Voler Systems provides comprehensive documentation compliance assistance, including support with regulatory submissions and guidance on meeting specific standards, helping startups in the medical equipment sector effectively address regulatory challenges. The duration for regulatory compliance can vary significantly, often taking several months to years, depending on the complexity of the item and the regulatory pathway. As Patti White, CEO of Hemex Health, noted, effective diagnosis can save 70% of children diagnosed with sickle cell disease, underscoring the importance of timely regulatory processes.
Production and Deployment: The final stage of the IoT product development process involves refining the design for mass production and then launching the product into the market. Successful deployment necessitates thorough preparation, including quality control measures and post-launch support to ensure the product performs effectively in real-world settings. Voler Systems is committed to delivering quality products on time and within budget, ensuring that each phase is crucial to confirming that the final product is secure, efficient, and meets the needs of healthcare providers and users alike.
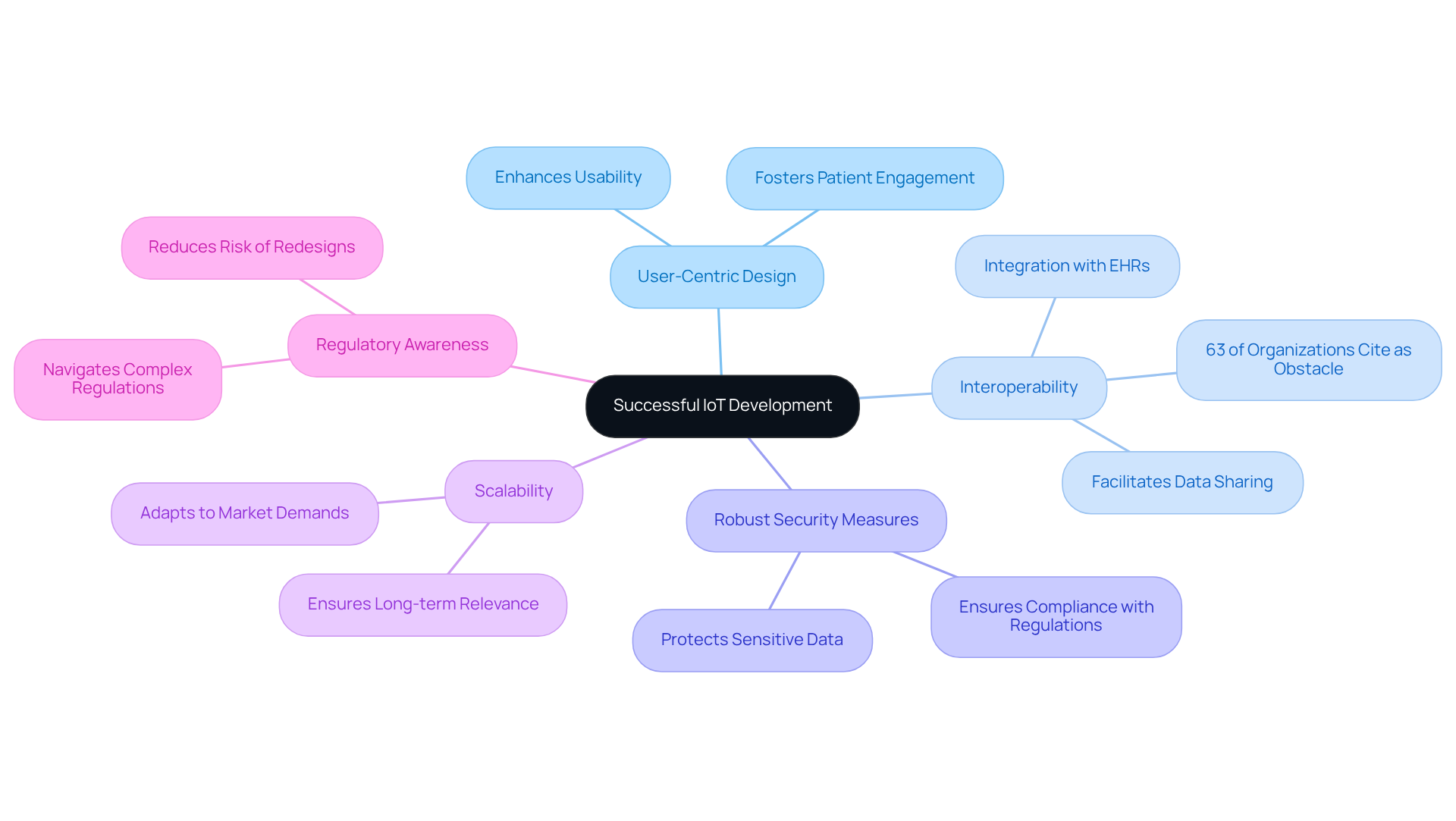
Successful IoT development in the medical device sector hinges on several essential attributes:
User-Centric Design: Prioritizing the needs and experiences of end-users, including individuals receiving care and healthcare providers, is crucial. A user-centered approach not only enhances usability but also fosters patient engagement, which is vital for the adoption of new technologies.
Interoperability: Seamless communication between equipment and other systems is paramount. Interoperability facilitates data sharing and integration, enabling healthcare providers to make informed decisions. However, challenges remain, with 63% of organizations referencing interoperability as a significant obstacle in IoT medical equipment. Successful instances comprise tools that connect with electronic health records (EHRs), enabling real-time data access and enhanced outcomes for individuals.
Robust Security Measures: Implementing strong security protocols is essential to protect sensitive client data and ensure compliance with regulations such as HIPAA. As the healthcare technology landscape evolves, the need for enhanced security measures becomes increasingly vital to prevent data breaches and uphold patient trust.
Scalability: Designing products that can adapt to changing market demands and technological advancements is vital for long-term success. Scalable solutions enable manufacturers to react swiftly to new opportunities and challenges, ensuring that products remain relevant in a rapidly evolving industry.
Regulatory Awareness: Navigating the complex regulatory landscape is crucial for ensuring compliance and facilitating market entry. Understanding the requirements set forth by regulatory bodies can significantly reduce the risk of costly redesigns and delays in product launches.
These characteristics are not only vital for the IoT product development process of innovative medical devices but also for ensuring their safety and effectiveness in real-world applications.
The IoT product development process for medical devices serves as a critical framework that guarantees the creation of innovative and compliant healthcare solutions. This process encompasses not only the technical aspects of development but also highlights the significance of user needs and regulatory standards, ultimately enhancing patient care and outcomes.
Key stages of the IoT product development process are essential, including:
Each phase plays a vital role in ensuring that the final product is both effective and secure, addressing the unique challenges presented by the healthcare sector. Furthermore, the discussion emphasizes crucial characteristics for successful IoT development, such as:
As the healthcare landscape continues to evolve, the adoption of the IoT product development process becomes increasingly significant. Stakeholders in the medical device industry are urged to embrace these best practices to effectively navigate the complexities of IoT integration. By doing so, they can enhance the efficiency and reliability of their products, ultimately contributing to improved health outcomes for patients worldwide.
What is the IoT product development process?
The IoT product development process is a structured framework designed to create, develop, and deploy Internet of Things (IoT) devices, particularly in the healthcare sector.
What are the critical stages of the IoT product development process?
The critical stages include ideation, prototyping, testing, and regulatory compliance.
Why is the IoT product development process important in healthcare?
It is important for mitigating risks, ensuring the final product meets user needs, and adhering to industry standards, especially given the stringent regulations governing healthcare instruments.
How does IoT technology enhance healthcare instruments?
IoT technology enhances monitoring capabilities, data collection, and overall patient care.
What is the projected impact of IoT on healthcare instruments by 2026?
It is projected that approximately 70% of healthcare instruments will incorporate IoT technology by 2026.
How does a well-defined IoT product development approach benefit medical IoT solutions?
A well-defined approach streamlines the development phases and bolsters the reliability and effectiveness of medical IoT solutions, ultimately leading to improved patient outcomes and satisfaction.
