4 Best Practices for Analog and Mixed Signal Circuit Design
Explore best practices in analog and mixed signal circuit design for optimal performance.
In the complex realm of medical device manufacturing, the design of printed circuit boards (PCBs) is a pivotal element in ensuring safety and efficacy. As regulatory standards tighten, manufacturers encounter the dual challenge of meeting compliance requirements while delivering high-quality, reliable products. This article explores four best practices that can enhance PCB design in medical applications, providing insights into:
As the stakes continue to rise, how can developers effectively navigate these complexities to not only meet but exceed industry expectations?
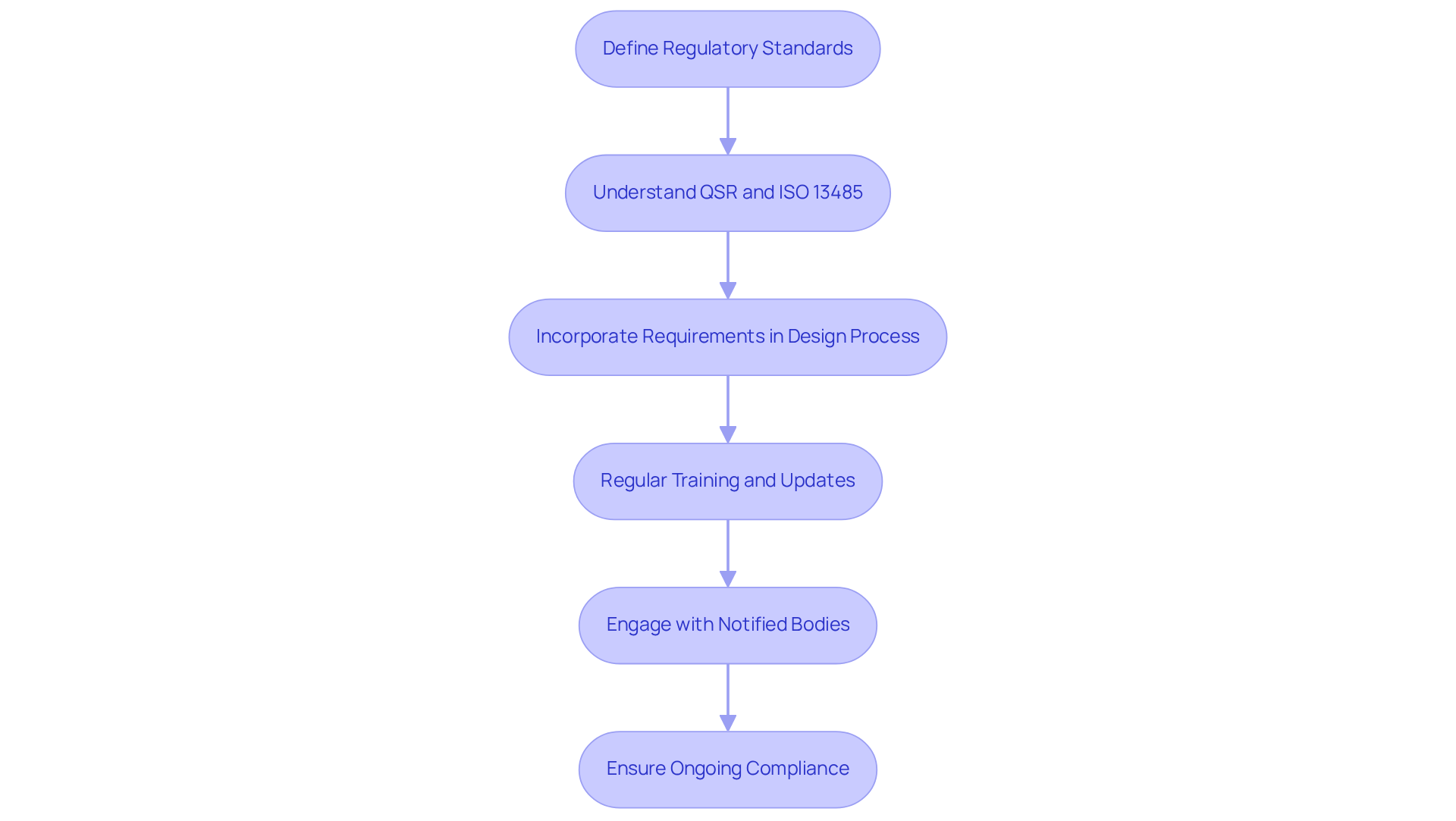
In the healthcare equipment sector, compliance with regulatory guidelines is essential for ensuring product safety and effectiveness. The FDA's Quality System Regulation (QSR) and ISO 13485 serve as foundational frameworks that dictate the requirements for quality management systems in medical device manufacturing. Adhering to these standards not only guarantees that the pcb design board layouts are reliable and efficient but also helps mitigate the risk of costly setbacks and potential patient injuries stemming from non-compliance.
As of early 2026, manufacturers must exercise heightened vigilance, as the QSR will transition to align with ISO 13485 requirements, emphasizing a risk-based approach to quality management. This shift necessitates that development teams incorporate these regulatory requirements from the outset of the creation process. Regular training and updates on regulatory changes should be integrated into the design team's routine to ensure ongoing compliance throughout the product lifecycle.
Case studies have shown that manufacturers who proactively engage with these guidelines, such as those supported by Voler Systems, experience enhanced product quality and improved market access. This underscores the significance of a robust compliance strategy in today's complex regulatory environment. Furthermore, early engagement with Notified Bodies is crucial to avoid compliance gaps and bottlenecks, facilitating a smoother transition to the new regulatory framework.
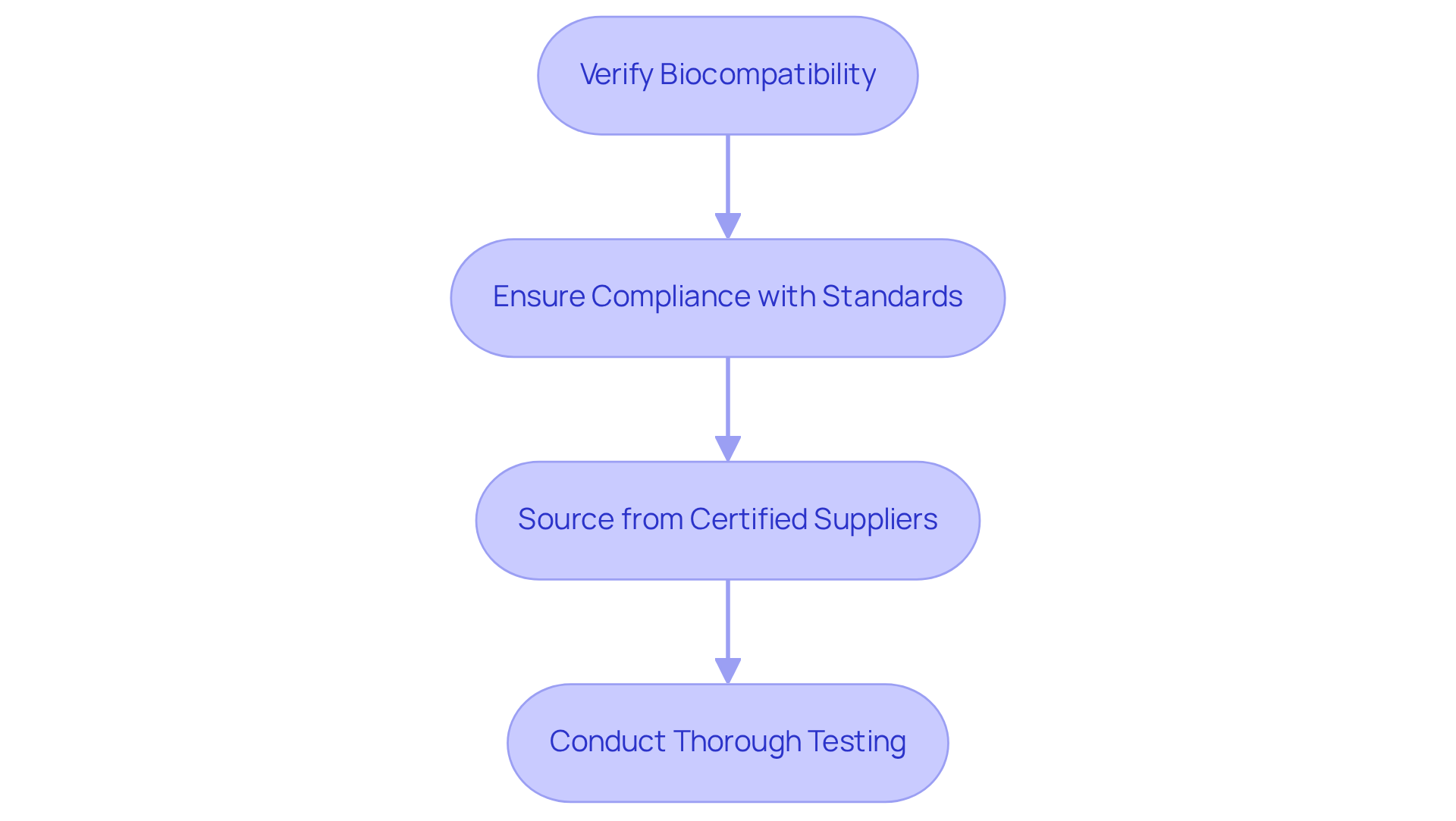
Choosing high-grade materials and components is essential for the effectiveness of healthcare device PCBs. These materials must fulfill electrical performance requirements and adhere to stringent biocompatibility standards. Frequently used materials, such as polyimide and FR-4, are preferred for their exceptional thermal and mechanical characteristics, making them suitable for healthcare applications. Furthermore, sourcing components from reputable suppliers who offer medical-grade certifications is crucial. This practice ensures that materials can withstand sterilization processes and the environmental conditions typical in medical settings.
To guarantee reliability and safety, Voler Systems conducts regular evaluations of suppliers and materials, reinforcing high criteria and mitigating risks associated with material failures. Testimonials from satisfied clients highlight the effectiveness of our compliance review process, ensuring that the products developed are both dependable and meet emissions and ESD criteria.
When selecting materials, consider the following steps:
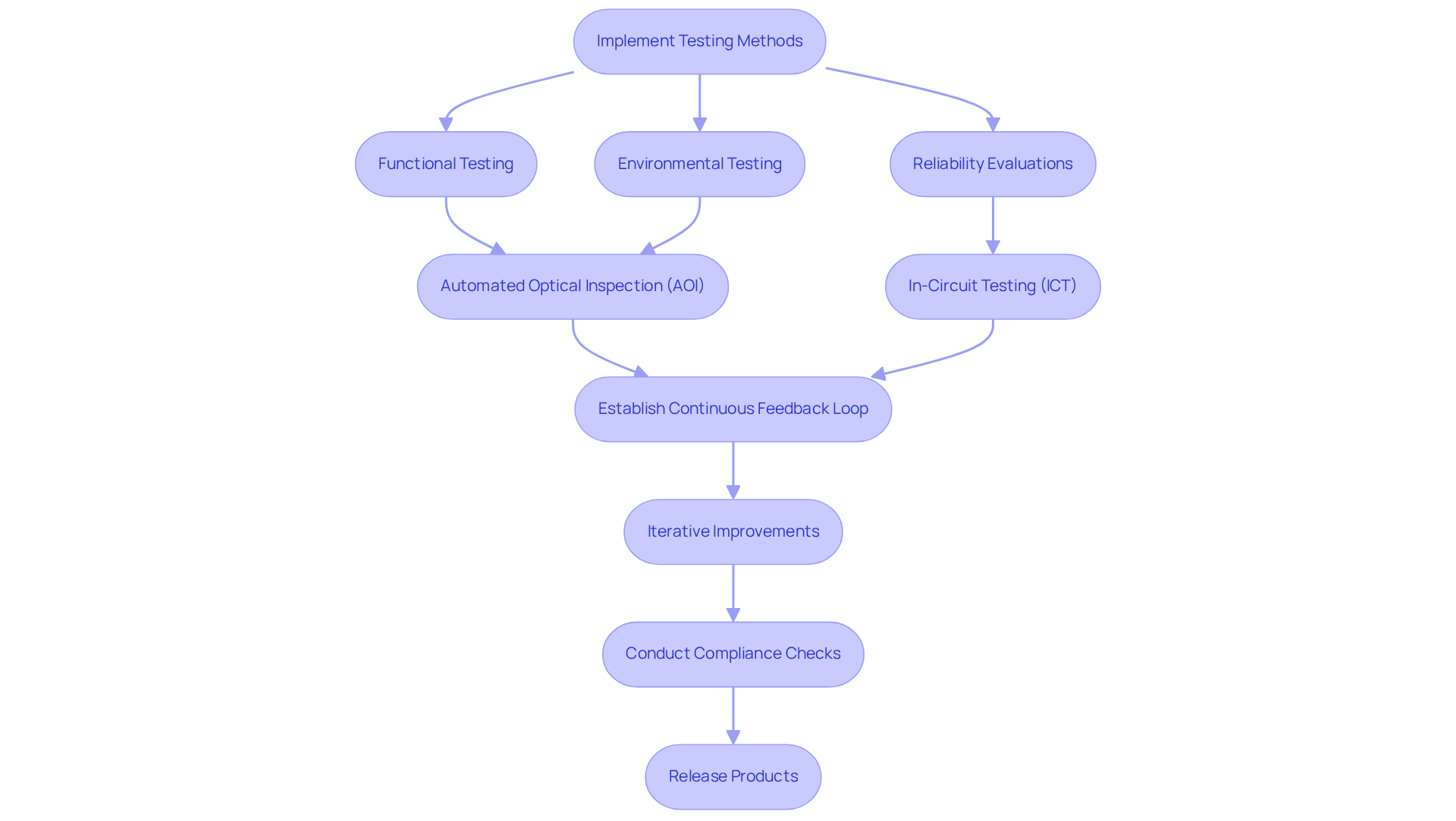
A robust testing and quality assurance framework is essential for healthcare PCBs, encompassing functional testing, environmental testing, and reliability evaluations. Implementing methods such as Automated Optical Inspection (AOI) and In-Circuit Testing (ICT) is vital for early defect detection, significantly mitigating the risk of costly recalls and compliance issues. For example, AOI can enhance defect detection accuracy, achieving up to 95% precision, which is critical in high-stakes environments like medical device manufacturing.
Establishing a continuous feedback loop between testing and design teams promotes iterative improvements, ensuring that any design flaws are promptly addressed. Moreover, quality assurance must incorporate rigorous compliance checks against regulatory standards, guaranteeing that all products meet the necessary safety and performance criteria prior to market release. This proactive approach not only enhances product reliability but also aligns with industry best practices, ultimately safeguarding patient health and improving overall market competitiveness.
Voler Systems offers expertise in navigating compliance challenges, particularly for startups in the healthcare equipment sector, ensuring that documentation and testing procedures adhere to regulatory standards. Additionally, recognizing common pitfalls in manufacturing tests is crucial for maintaining quality and efficiency in electronic product development, and Voler Systems provides best practices to mitigate these challenges.
Collaboration between creative and manufacturing teams is essential for the successful development of medical device PCB design boards. Engaging manufacturing engineers early in the PCB design board phase enables the identification of potential production challenges, facilitating a smoother transition from the PCB design board to manufacturing. This proactive involvement can significantly influence project timelines, reducing delays and enhancing overall efficiency.
To strengthen communication, the use of collaborative tools and platforms is vital, ensuring that all team members remain aligned on project specifications and timelines. Regular meetings and updates promote a culture of transparency and accountability, which is crucial for maintaining momentum and achieving high-quality outcomes.
By integrating diverse skills and perspectives, teams can manage complexities more effectively, ultimately leading to innovative solutions that comply with stringent regulatory requirements. For example, Voler Systems has successfully implemented these practices in previous projects, demonstrating their commitment to quality and compliance through rigorous reviews that ensure adherence to emissions and ESD standards.
Effective PCB design in medical devices relies heavily on a thorough understanding of regulatory standards, material quality, rigorous testing, and collaborative processes. By prioritizing compliance with frameworks such as the FDA's Quality System Regulation and ISO 13485, manufacturers can ensure that their products not only meet safety requirements but also enhance overall patient outcomes. The shift towards a risk-based approach highlights the necessity of integrating these standards from the very beginning of the design process.
Key practices discussed in this article include:
Each of these components is vital in mitigating risks, enhancing product reliability, and streamlining the development process. The insights presented underscore that proactive engagement with compliance and quality measures leads to improved market access and a stronger competitive advantage.
Ultimately, the importance of these best practices cannot be overstated. As the medical device landscape continues to evolve, adopting these strategies will not only protect patient health but also ensure that companies remain at the forefront of innovation and compliance. By committing to excellence in PCB design, manufacturers can contribute to a safer and more effective healthcare environment.
What are the key regulatory standards in the healthcare equipment sector?
The key regulatory standards include the FDA's Quality System Regulation (QSR) and ISO 13485, which dictate the requirements for quality management systems in medical device manufacturing.
Why is compliance with regulatory guidelines important in healthcare equipment manufacturing?
Compliance ensures product safety and effectiveness, mitigates the risk of costly setbacks, and helps prevent potential patient injuries stemming from non-compliance.
What significant change will occur in early 2026 regarding the QSR?
In early 2026, the QSR will transition to align with ISO 13485 requirements, emphasizing a risk-based approach to quality management.
How should development teams prepare for the upcoming changes in regulatory requirements?
Development teams should incorporate regulatory requirements from the outset of the creation process and integrate regular training and updates on regulatory changes into their routine.
What benefits do manufacturers experience by engaging proactively with regulatory guidelines?
Manufacturers that proactively engage with regulatory guidelines often experience enhanced product quality and improved market access.
Why is early engagement with Notified Bodies important?
Early engagement with Notified Bodies is crucial to avoid compliance gaps and bottlenecks, facilitating a smoother transition to the new regulatory framework.
