10 Essential Elements of Product Design for Medical Devices
Explore the essential elements of product design for effective medical devices and...
In the rapidly evolving landscape of healthcare technology, the components that constitute medical devices have become increasingly critical. Manufacturers are tasked with navigating a complex interplay of design, functionality, and regulatory compliance to develop products that not only adhere to stringent standards but also enhance patient care. This article explores the ten essential components for medical device manufacturers, emphasizing the innovations and strategies that can significantly improve device performance and reliability. Given the multitude of factors at play, how can manufacturers ensure that their products remain both cutting-edge and compliant in an increasingly competitive market?
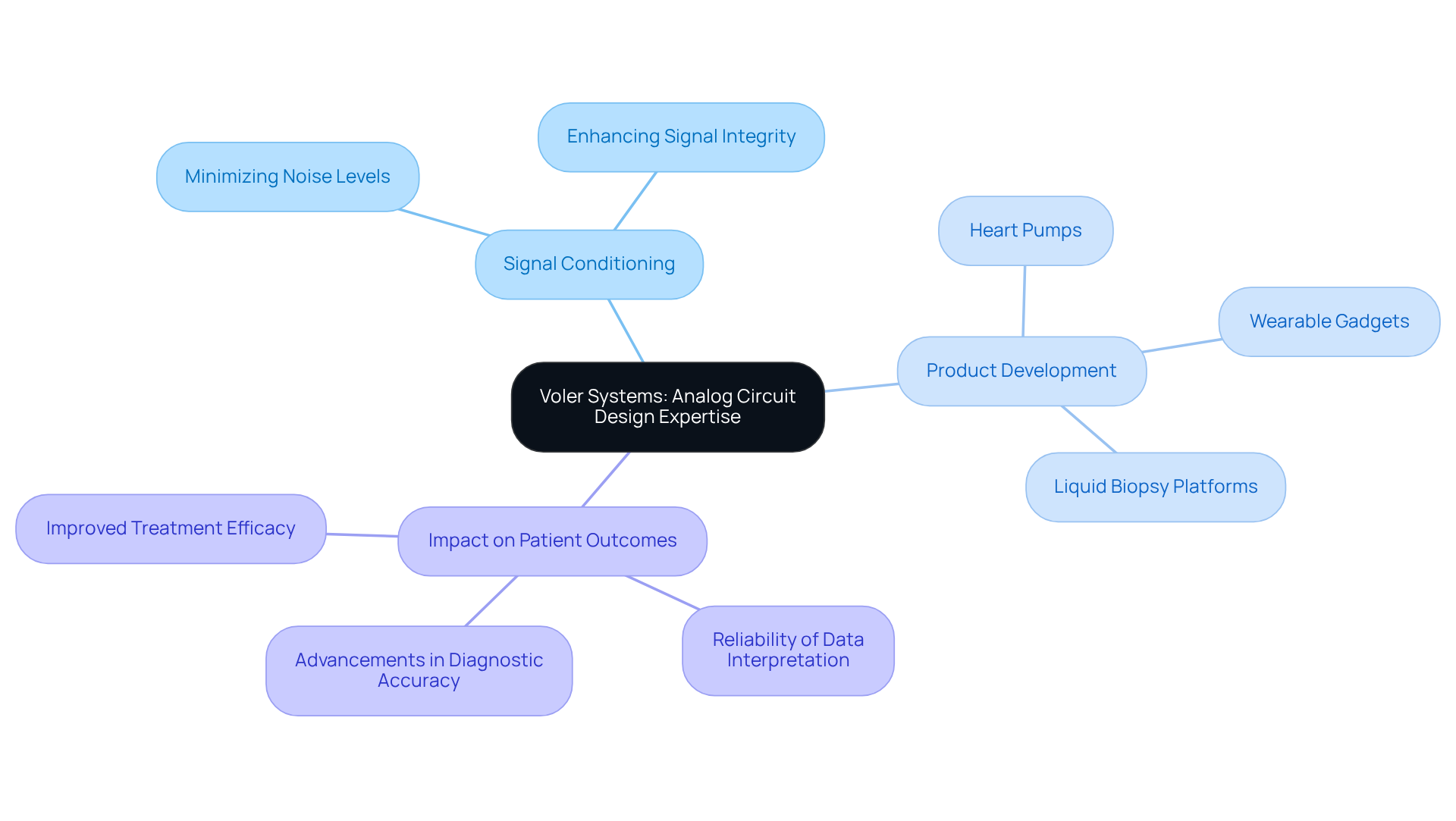
Voler Systems stands out in analog circuit design, focusing on the precise conditioning of signals from various sensors, which is crucial for the functionality of healthcare instruments. Our expertise encompasses the development of different parts of a product, such as wearable gadgets, heart pumps, and liquid biopsy platforms, utilizing AI-assisted engineering to expedite product development. The accuracy of signal conditioning has a direct impact on patient outcomes, ensuring that instruments interpret data with reliability. Effective analog design significantly minimizes noise levels, thereby enhancing the clarity of physiological signals. This capability is vital in environments where equipment must operate under diverse conditions, maximizing signal integrity and operational reliability.
Successful projects, such as the creation of wearable sensors and heart monitoring systems, illustrate how Voler Systems employs advanced analog techniques to develop devices that adhere to stringent industry standards and elevate patient care. By emphasizing signal conditioning and integrating ultra-low power design strategies, Voler Systems plays a pivotal role in advancing innovative medical technologies that improve diagnostic accuracy and treatment efficacy.
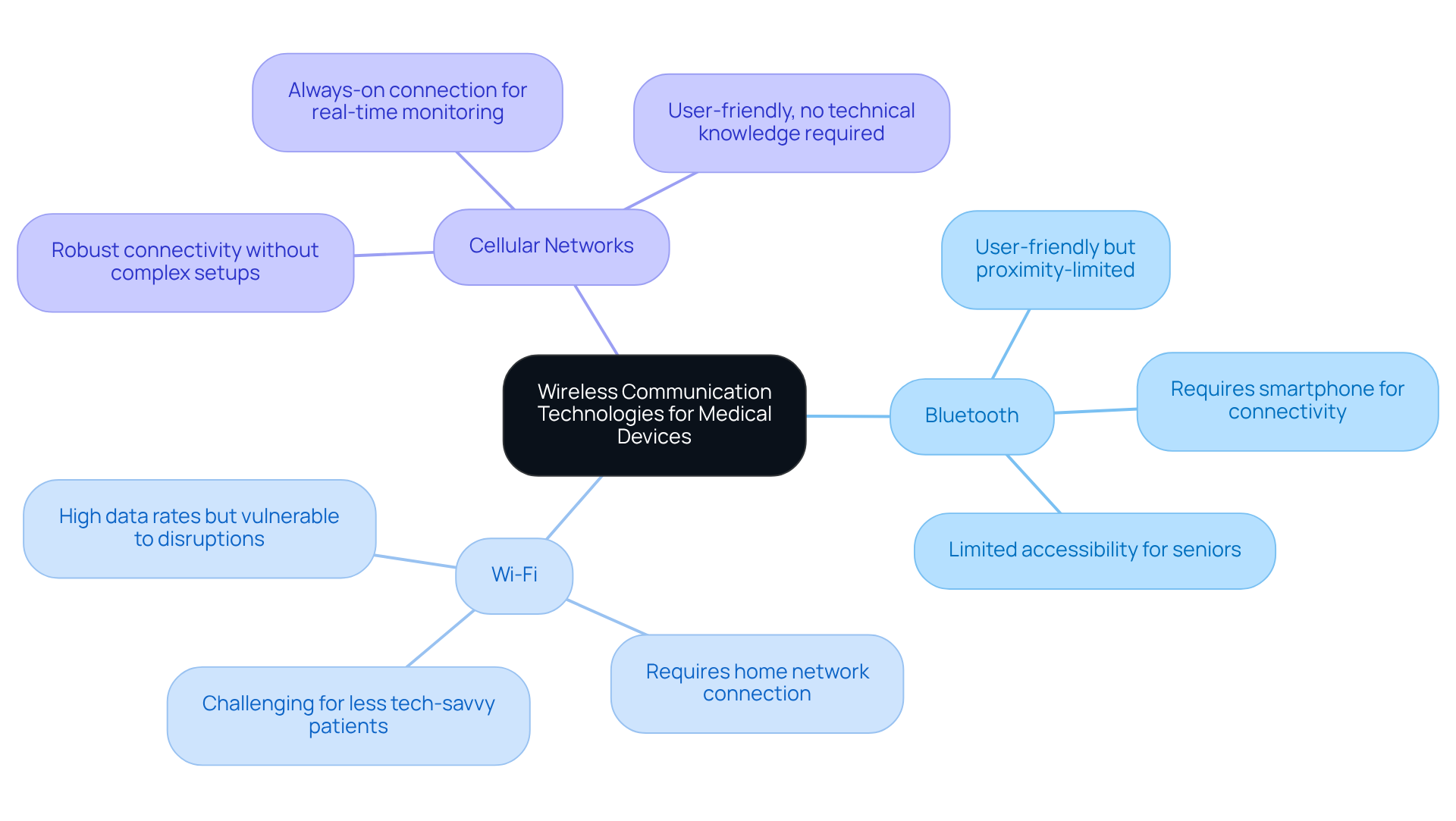
Wireless communication technologies, such as Bluetooth, Wi-Fi, and cellular networks, are crucial for the functionality of modern medical devices. These innovations enable real-time data transfer, which is vital for remote patient monitoring and timely healthcare interventions. The choice of wireless protocols significantly impacts performance, reliability, and compliance with regulatory standards.
Currently, the market share for Bluetooth and Wi-Fi in medical devices indicates a growing preference for cellular technology, attributed to its reliability and user-friendliness. Effective integration of these technologies requires thorough coexistence testing to mitigate interference risks and enhance performance across various environments. Voler Systems is dedicated to seamlessly integrating these wireless solutions into the parts of a product in healthcare, thereby ensuring effective communication and improved patient care.
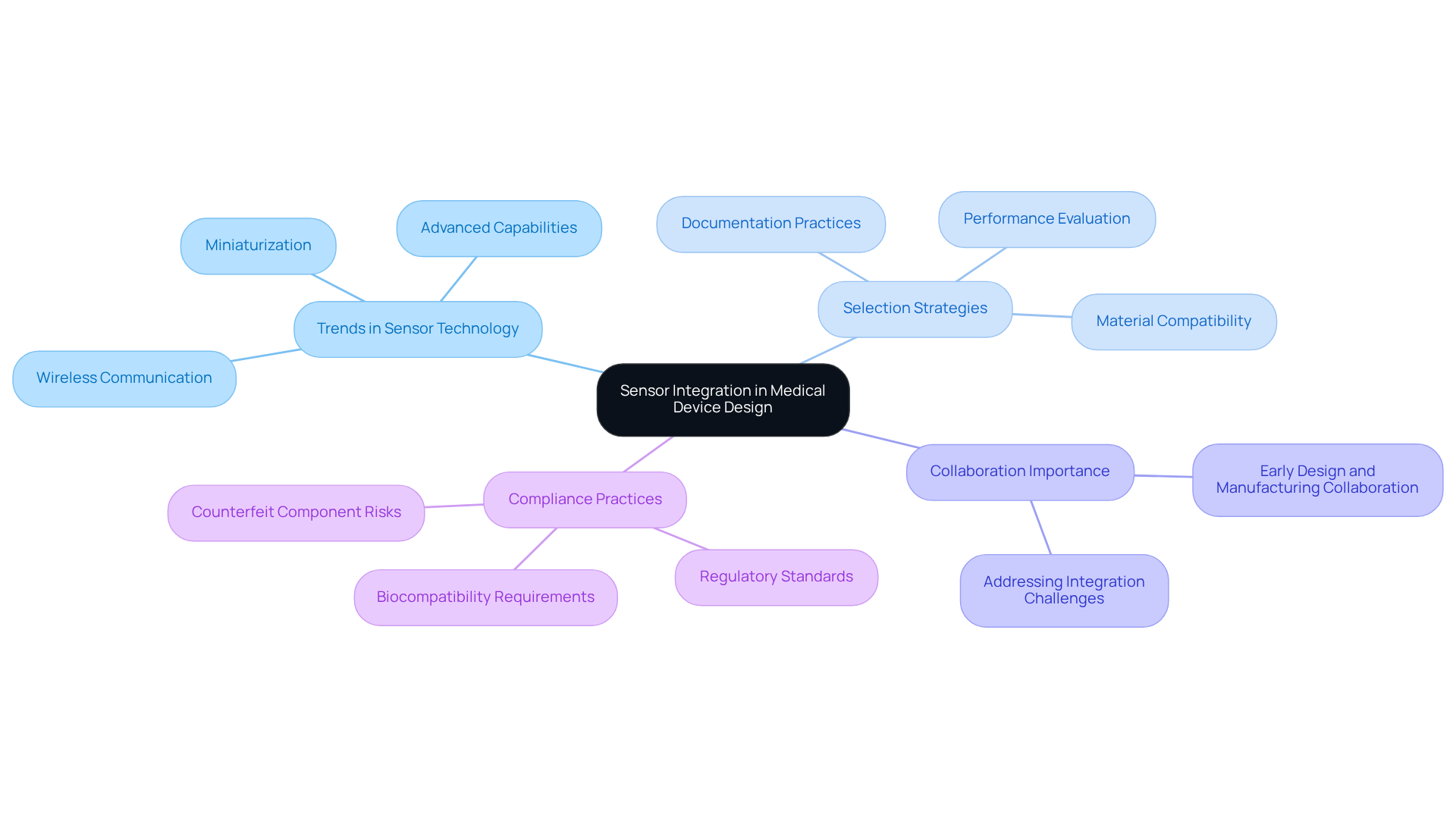
Incorporating sensors into healthcare instruments is essential for gathering critical health information and enhancing patient support. The selection of appropriate sensors must take into account compatibility with the device's architecture, ensuring seamless functionality within the parts of a product. Current trends in sensor technology for 2026 highlight miniaturization, wireless communication, and advanced capabilities, which are vital for the development of innovative medical solutions.
Voler Systems employs a systematic approach to sensor integration, prioritizing accuracy, reliability, and user-friendliness. Successful projects underscore the significance of early collaboration between design and manufacturing teams to address potential integration challenges. For example, the development of sensor-enabled catheters illustrates that meticulous attention to sensor placement and material compatibility is crucial for accurate data collection and patient safety.
Optimal strategies for selecting sensors in healthcare equipment design involve evaluating their performance under various environmental conditions and ensuring compliance with regulatory standards. Furthermore, manufacturers should adopt rigorous documentation practices, such as maintaining comprehensive records of sensor specifications and testing results, to support compliance efforts. As sensor technology continues to advance, manufacturers must remain vigilant regarding the parts of a product to ensure component compatibility, avoid production interruptions, and maintain equipment efficacy. By leveraging these insights, healthcare equipment producers can enhance the functionality and reliability of their products, ultimately leading to improved patient outcomes.
Creating software and firmware for healthcare equipment necessitates strict adherence to regulatory standards, particularly IEC 62304, which governs the lifecycle of healthcare software. This standard is vital for ensuring that software is safe, effective, and reliable. Voler Systems promotes a comprehensive software development lifecycle that integrates essential parts of a product, including risk management, validation, and verification processes. By embedding compliance considerations from the initial phases, manufacturers can adeptly navigate the complexities of regulatory mandates, ultimately delivering high-quality healthcare products that incorporate the essential parts of a product to meet industry standards.
To enhance compliance with IEC 62304, manufacturers should consider the following critical steps:
Successful adherence to IEC 62304 not only enhances the safety of the parts of a product but also fosters trust among stakeholders, ensuring that products are both innovative and reliable in meeting patient needs.
Motion control systems are essential in the operation of various medical devices, including surgical robots and infusion pumps. These systems must be designed with precision and reliability to ensure patient safety. Voler Systems employs advanced motion control technologies, such as Edge AI, to enhance performance by minimizing errors and optimizing response times. Through the integration of sophisticated control algorithms and AI innovations, they ensure that healthcare instruments operate seamlessly and efficiently.
Best practices in the design of reliable motion control systems involve rigorous testing and validation to comply with stringent regulatory standards. This approach guarantees that instruments not only perform accurately but also uphold high levels of safety and efficacy in patient care.
Field Programmable Gate Arrays (FPGAs) offer exceptional adaptability in the design of medical equipment, enabling engineers to customize hardware for specific applications. This adaptability is essential for devices that require real-time data processing, such as imaging systems and diagnostic tools.
For example, a systems company has effectively implemented FPGA methods across various projects, achieving a remarkable 144× speed increase compared to traditional CPUs for faster dose calculations and imaging adjustments. This enhancement is critical in high-stakes applications like MRI, CT, and ultrasound systems.
By harnessing FPGA technology, Voler Systems develops tailored solutions that not only improve performance but also comply with stringent regulatory standards. Their proficiency in FPGA development ensures that healthcare instruments can adapt to technological advancements, employing advanced image enhancement techniques such as noise reduction and edge detection to boost diagnostic accuracy.
Furthermore, FPGAs provide a competitive advantage over GPUs in real-time dose adjustments and imaging for radiation therapy, solidifying their role as essential parts of a product in modern healthcare equipment.
Embedded systems play a crucial role in the functionality of modern healthcare instruments, enabling them to execute complex tasks with precision and efficiency. These systems seamlessly integrate the parts of a product, including hardware and software components, allowing for real-time processing and effective data management. Voler Systems specializes in developing robust embedded systems specifically designed for medical applications, ensuring high reliability and performance. A significant focus on power efficiency is vital, particularly for battery-operated devices, as it directly influences their operational longevity and user satisfaction.
Current trends in embedded system design highlight the importance of modularity and scalability in the parts of a product, which enables manufacturers to respond to the rapidly evolving healthcare landscape. Successful designs prioritize interoperability, ensuring that equipment can communicate effectively with hospital systems and cloud platforms, which is essential for integrated healthcare solutions. By leveraging advanced technologies and adhering to established best practices, the company supports manufacturers in developing innovative healthcare solutions that meet the demands of today's health environment.
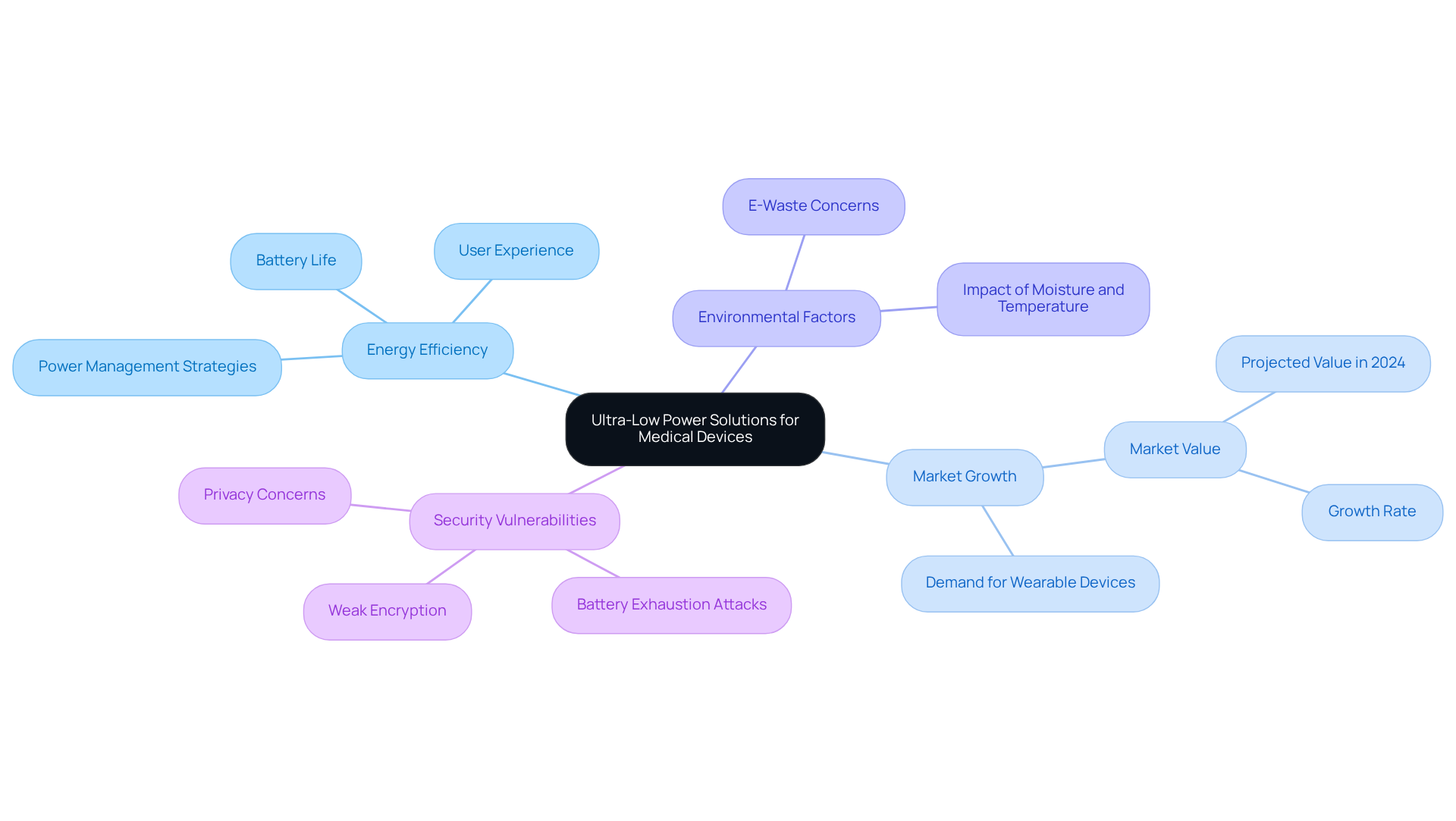
Ultra-low power design is crucial for healthcare devices that depend on batteries, particularly wearable health monitors. By integrating energy-efficient components and optimizing power management strategies, companies can enable products to function for extended periods without the need for frequent recharging. This strategy not only improves user experience but also significantly enhances patient compliance. As the demand for prolonged battery life escalates in 2026, energy efficiency in wearable health monitors becomes increasingly essential.
Voler Systems' expertise in ultra-low power solutions positions them at the forefront of developing sustainable medical innovations, ensuring that devices align with the evolving requirements of both manufacturers and users. Environmental factors, such as moisture and temperature, can greatly affect the performance and longevity of wearable sensors, underscoring the necessity for robust energy management solutions. The global Wearable Medical Devices Market, projected to be valued at $42.1 billion in 2024, underscores the growing significance of these innovations.
Moreover, vulnerabilities such as battery exhaustion attacks highlight the urgent need for advanced energy management strategies. As industry experts have noted, "The unexpected COVID-19 pandemic has brought to the spotlight the use of wearable sensing technologies, positively shifting the perception and adoption of wearable technologies despite their privacy and security issues." This context underscores the critical importance of energy efficiency in the ongoing advancement of healthcare instruments.
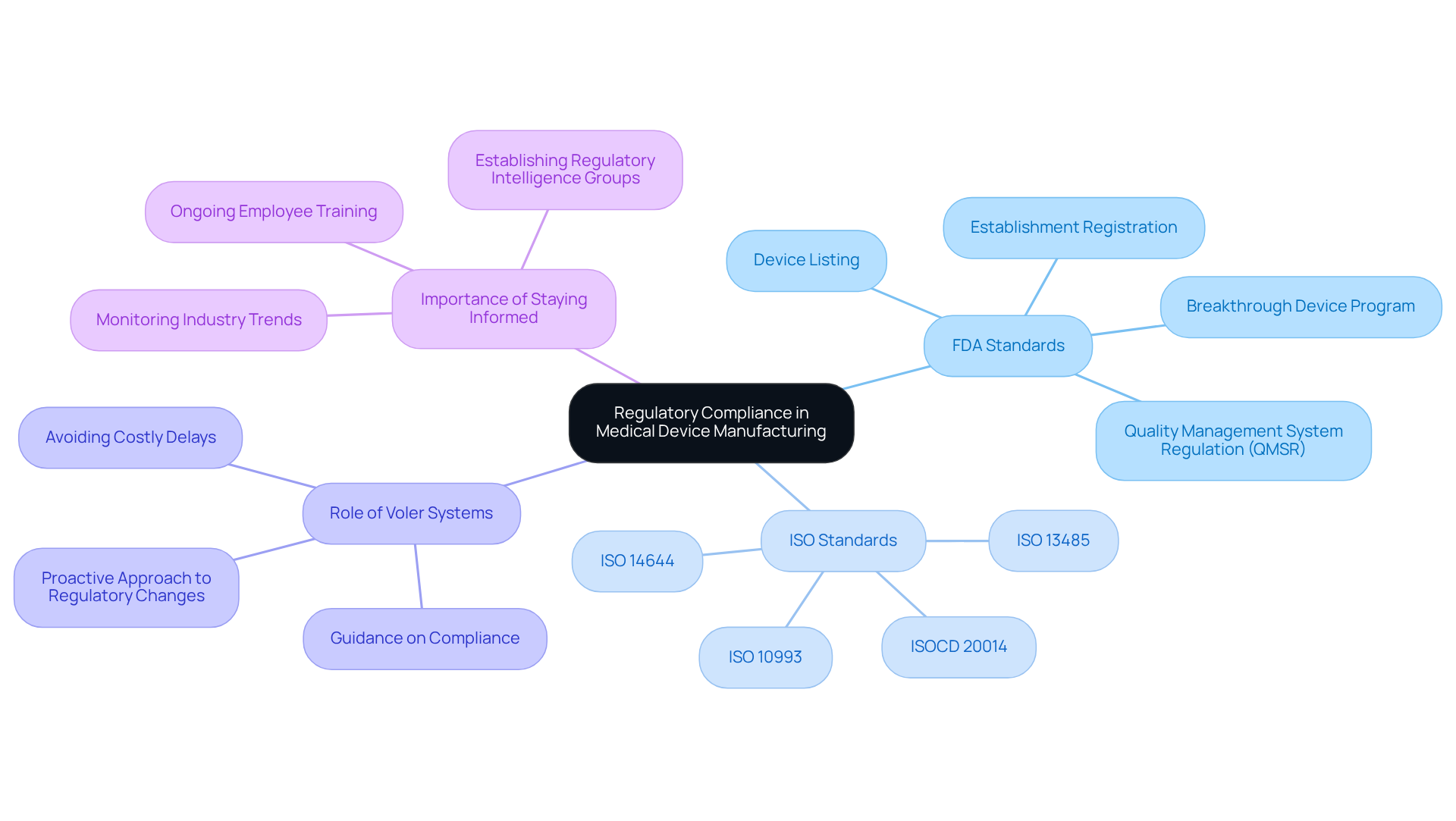
Navigating the regulatory landscape presents a significant challenge for medical device manufacturers, making compliance with FDA and ISO standards essential for ensuring product safety and efficacy. As we approach 2026, adherence to ISO 13485 remains a global benchmark for quality management, while the FDA's Quality Management System Regulation (QMSR) aligns with these standards to facilitate smoother market access. This evolving regulatory environment highlights the necessity of staying informed about industry trends and compliance requirements.
Voler Systems plays a crucial role in guiding clients through these complex regulatory requirements, helping them avoid costly delays and ensuring efficient market entry. Their expertise aids in understanding the nuances of compliance and positions manufacturers for long-term success by fostering a proactive approach to regulatory changes. By leveraging their knowledge, companies can navigate the complexities of FDA regulations, including establishment registration and product listing, while maintaining high-quality standards that meet rigorous industry requirements. This comprehensive support is vital for producers aiming to develop innovative healthcare products that comply with evolving regulatory standards.
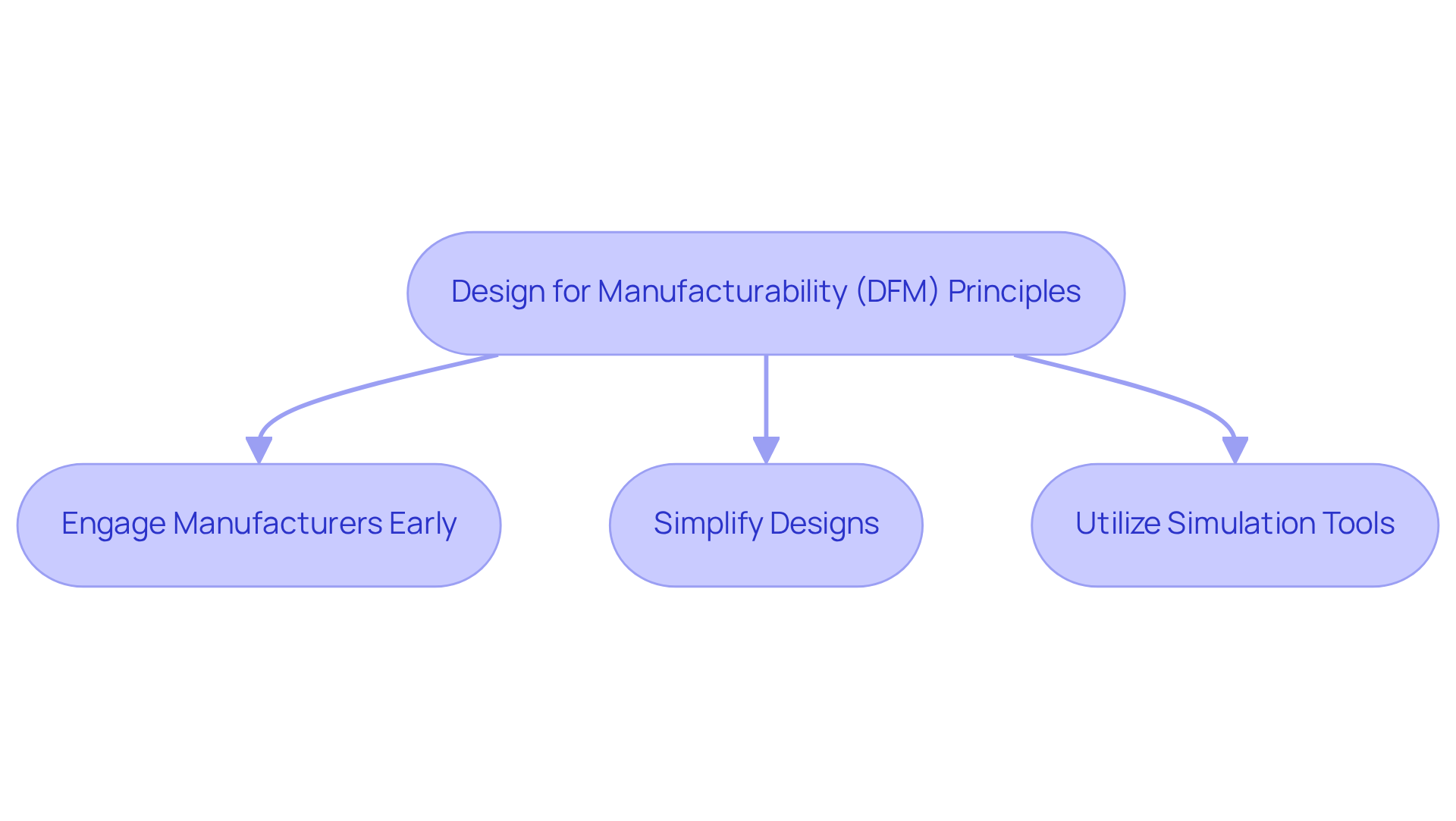
Designing medical devices with manufacturability as a primary focus is essential for improving production efficiency and cost-effectiveness. By applying design for manufacturability (DFM) principles, Voler Systems enhances the production process, streamlining designs and minimizing potential errors. This proactive strategy involves close collaboration with manufacturers, ensuring that devices are not only functional but also scalable for mass production.
To optimize the DFM process, consider the following best practices:
This emphasis on manufacturability accelerates time-to-market and improves product quality, aligning with industry trends that prioritize efficient and effective healthcare solutions.
As the medical device landscape evolves in 2026, adopting DFM principles will be crucial for manufacturers striving to meet stringent regulatory standards while maintaining a competitive edge.
The landscape of medical device manufacturing is characterized by a range of essential components that collectively enhance patient care and ensure compliance with industry standards. Each element, from advanced analog circuit design to effective wireless communication technologies, plays a critical role in the functionality and reliability of healthcare instruments. By understanding and integrating these components, manufacturers can develop innovative solutions that not only meet regulatory requirements but also improve patient outcomes.
Key insights from this discussion underscore the importance of:
Technologies such as FPGA development and ultra-low power solutions are transforming the capabilities of medical devices, enabling real-time data processing and extended operational life. Moreover, prioritizing manufacturability through design for manufacturability principles ensures that these devices can be produced efficiently and effectively, aligning with the evolving demands of the healthcare industry.
As the medical technology landscape continues to evolve, embracing these essential components will be vital for manufacturers aiming to deliver safe, reliable, and innovative healthcare solutions. The ongoing commitment to integrating cutting-edge technologies and adhering to stringent compliance standards will not only enhance the quality of medical devices but also significantly impact patient care and safety. Engaging with these insights can inspire proactive strategies that elevate the standards of medical device manufacturing in the years ahead.
What is Voler Systems' expertise in analog circuit design?
Voler Systems specializes in analog circuit design, focusing on precise signal conditioning from various sensors, which is essential for healthcare instruments. Their expertise includes developing wearable gadgets, heart pumps, and liquid biopsy platforms using AI-assisted engineering to expedite product development.
How does signal conditioning impact patient outcomes?
The accuracy of signal conditioning directly affects patient outcomes by ensuring that instruments reliably interpret data. Effective analog design minimizes noise levels, enhancing the clarity of physiological signals, which is crucial in diverse operational environments.
What types of projects has Voler Systems successfully completed?
Voler Systems has successfully developed projects such as wearable sensors and heart monitoring systems, utilizing advanced analog techniques to create devices that meet stringent industry standards and improve patient care.
What are the key wireless communication technologies used in medical devices?
Key wireless communication technologies for medical devices include Bluetooth, Wi-Fi, and cellular networks. These technologies enable real-time data transfer, which is vital for remote patient monitoring and timely healthcare interventions.
What are the advantages and disadvantages of Bluetooth and Wi-Fi in medical devices?
Bluetooth is user-friendly but often requires proximity to a smartphone, limiting accessibility for some patients. Wi-Fi offers higher data transfer rates but can be disrupted by power failures or interference. Cellular networks provide robust connectivity without complex setups, making them suitable for critical monitoring applications.
What trends are emerging in sensor technology for medical devices?
Current trends in sensor technology for 2026 include miniaturization, wireless communication, and advanced capabilities, which are essential for developing innovative medical solutions.
How does Voler Systems approach sensor integration in medical device design?
Voler Systems employs a systematic approach to sensor integration, prioritizing accuracy, reliability, and user-friendliness. They emphasize early collaboration between design and manufacturing teams to address potential integration challenges.
What strategies should manufacturers adopt for selecting sensors in healthcare equipment design?
Manufacturers should evaluate sensor performance under various environmental conditions, ensure compliance with regulatory standards, and maintain comprehensive documentation of sensor specifications and testing results to support compliance efforts.
Why is sensor placement and material compatibility important in medical devices?
Proper sensor placement and material compatibility are crucial for accurate data collection and patient safety, as demonstrated in the development of sensor-enabled catheters.
How can healthcare equipment producers enhance product functionality and reliability?
By leveraging insights from sensor technology advancements and ensuring component compatibility, healthcare equipment producers can enhance the functionality and reliability of their products, ultimately leading to improved patient outcomes.
