Introduction
Field-Programmable Gate Arrays (FPGAs) are revolutionizing medical technology, significantly altering the healthcare landscape through their capacity for real-time data processing and adaptability to specific requirements. As the demand for precision and efficiency in medical devices escalates, it becomes essential for developers to grasp best practices in FPGA engineering to drive innovation.
However, engineers face considerable challenges in navigating the complex regulatory environment and ensuring compliance while integrating advanced technologies such as artificial intelligence.
To fully leverage the potential of FPGAs, what strategies can engineers implement to overcome these hurdles?
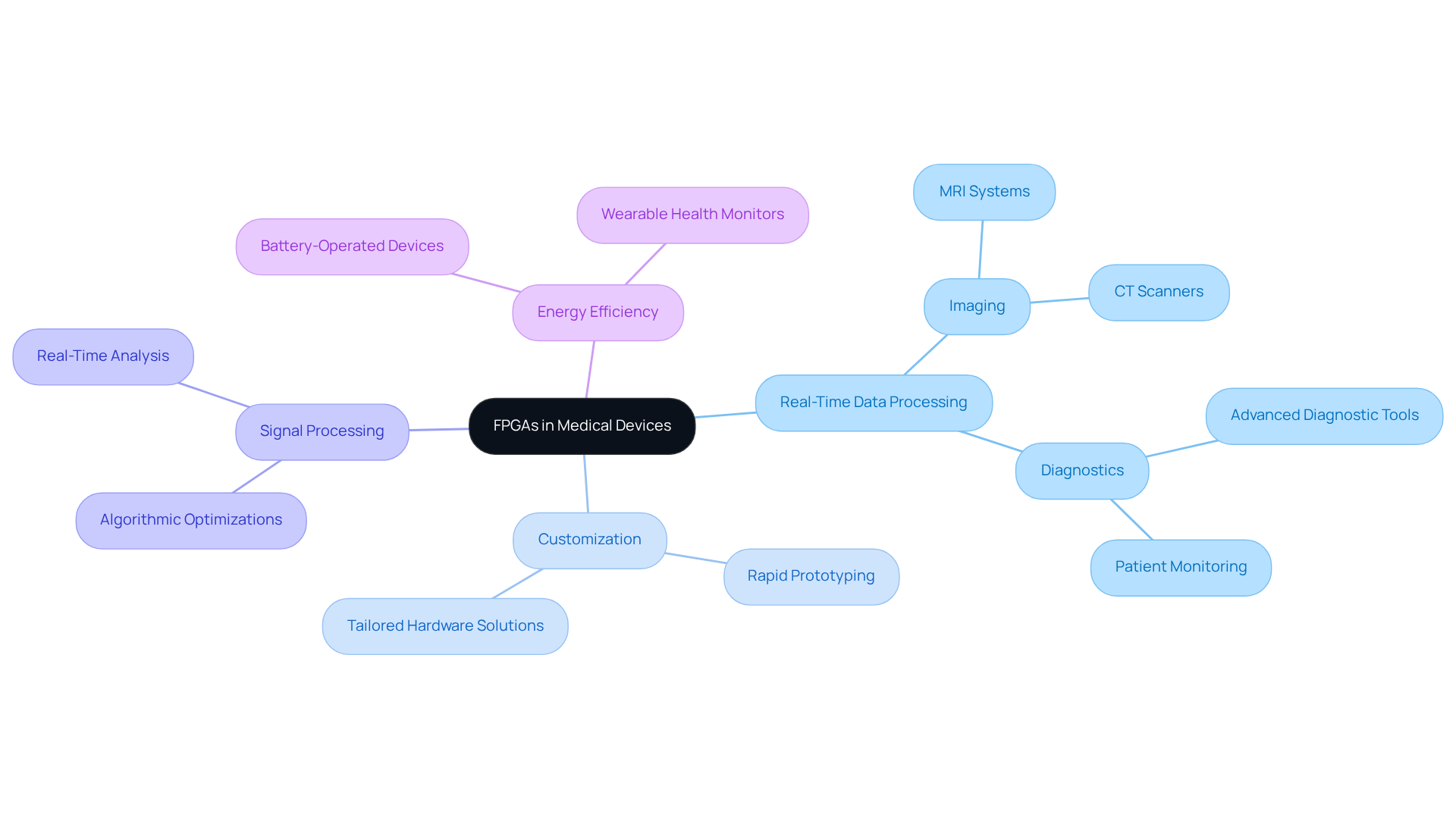
Understand the Role of FPGA in Medical Devices
Field-Programmable Gate Arrays (FPGAs) are revolutionizing medical equipment by enabling real-time data processing, which is essential for applications such as imaging, diagnostics, and patient monitoring. Their reconfigurable architecture empowers engineers to customize hardware to meet specific requirements, thereby facilitating rapid prototyping and iterative design.
For instance, FPGAs play a critical role in executing complex algorithms for signal processing in MRI machines, significantly improving image clarity and diagnostic accuracy. Additionally, their energy efficiency is particularly advantageous for battery-operated devices, extending operational life without sacrificing performance.
As the healthcare sector increasingly emphasizes precision and adaptability, a comprehensive understanding of FPGAs is vital for engineers and developers aiming to foster innovation in this domain.
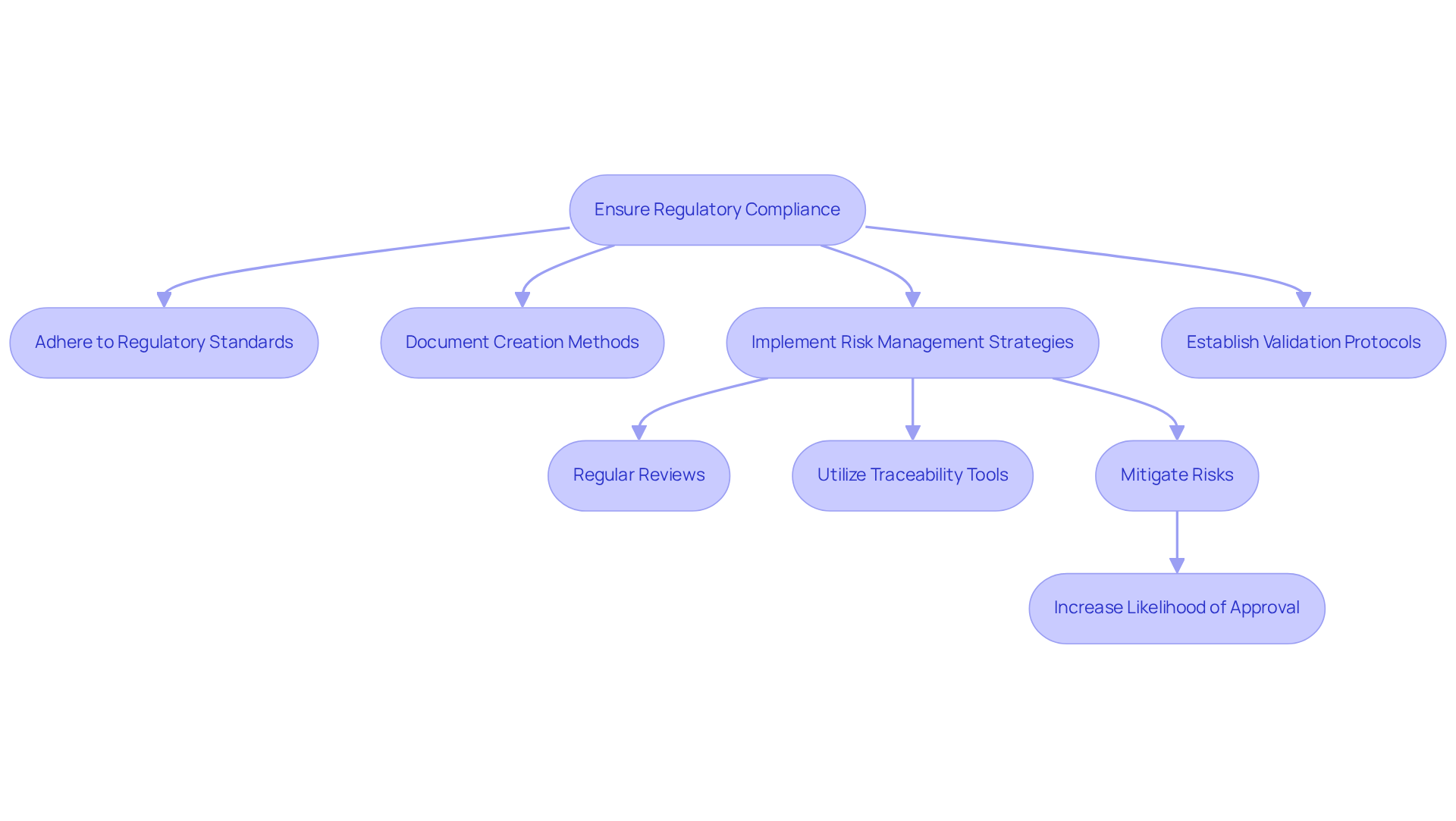
Ensure Regulatory Compliance in FPGA Design
Adherence to regulatory standards is essential in the medical device sector, particularly for FPGA engineering applications. Engineers must ensure compliance with guidelines established by regulatory bodies such as the FDA and IEC. This necessitates thorough documentation of creation methods, robust risk management strategies, and comprehensive validation protocols.
For instance, implementing a control framework that includes regular reviews and updates can significantly enhance compliance throughout the development lifecycle. Additionally, utilizing tools that ensure traceability of requirements to specification documents can streamline the compliance process. By prioritizing regulatory adherence from the outset, teams can effectively mitigate risks and increase the likelihood of successful product approval.
Statistics indicate that deficiencies in control systems were a primary reason for FDA Warning Letters, with 25 issued in fiscal year 2025. This underscores the importance of stringent compliance measures. Adopting these best practices not only aligns with FDA guidelines but also positions companies for success in navigating the complex regulatory landscape.
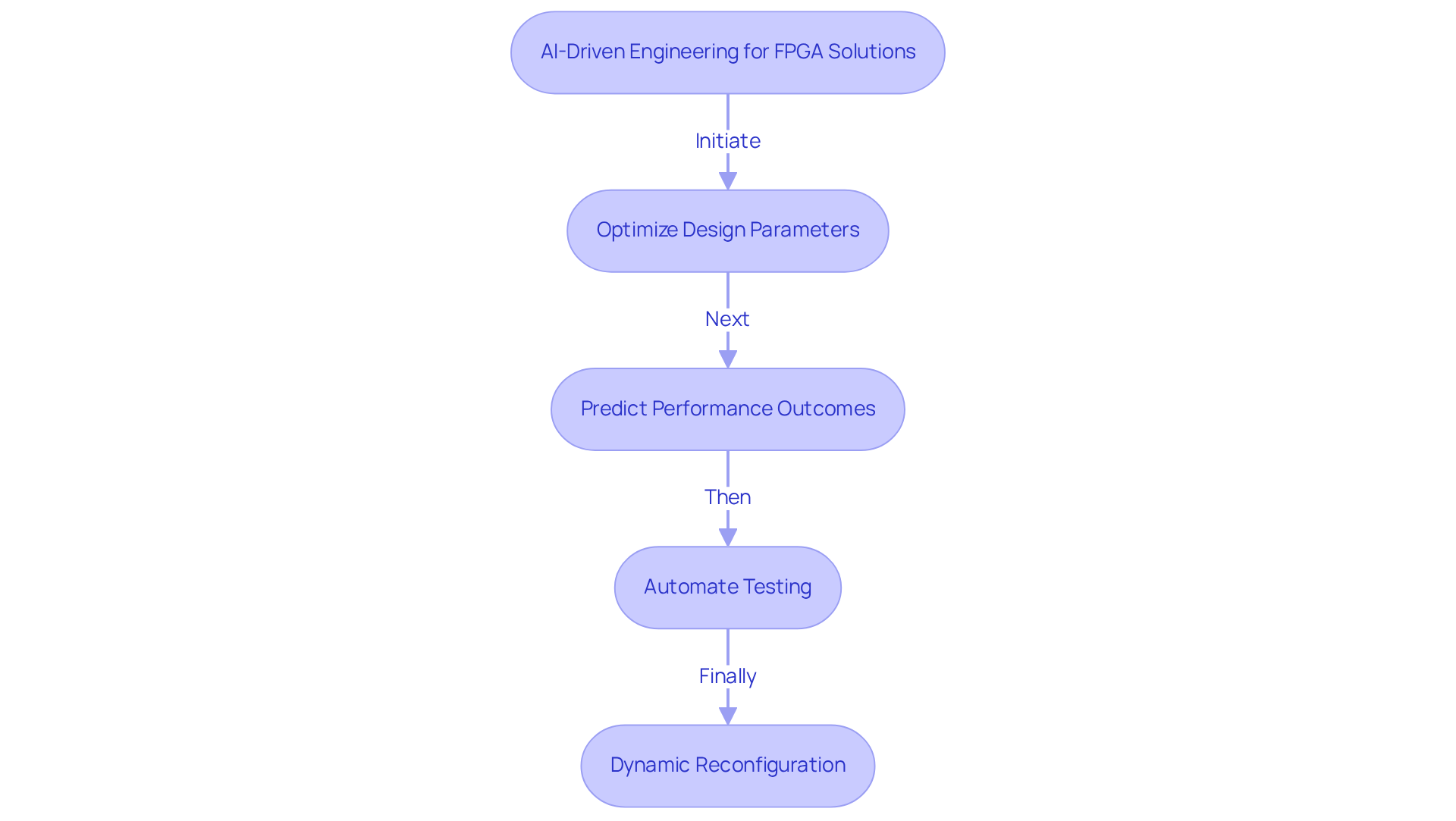
Leverage AI-Driven Engineering for Enhanced FPGA Solutions
The functionalities of medical equipment are markedly enhanced by incorporating AI into FPGA engineering development processes. AI algorithms optimize design parameters, predict performance outcomes, and automate testing, leading to faster development cycles and improved product quality. For instance, machine learning techniques analyze extensive datasets from clinical trials, enabling engineers to refine FPGA configurations based on real-world performance data. Additionally, AI facilitates dynamic reconfiguration of FPGAs during operation, allowing systems to adapt to changing conditions or user needs in real-time. By embracing AI-driven engineering, teams can push the boundaries of innovation in healthcare product development.
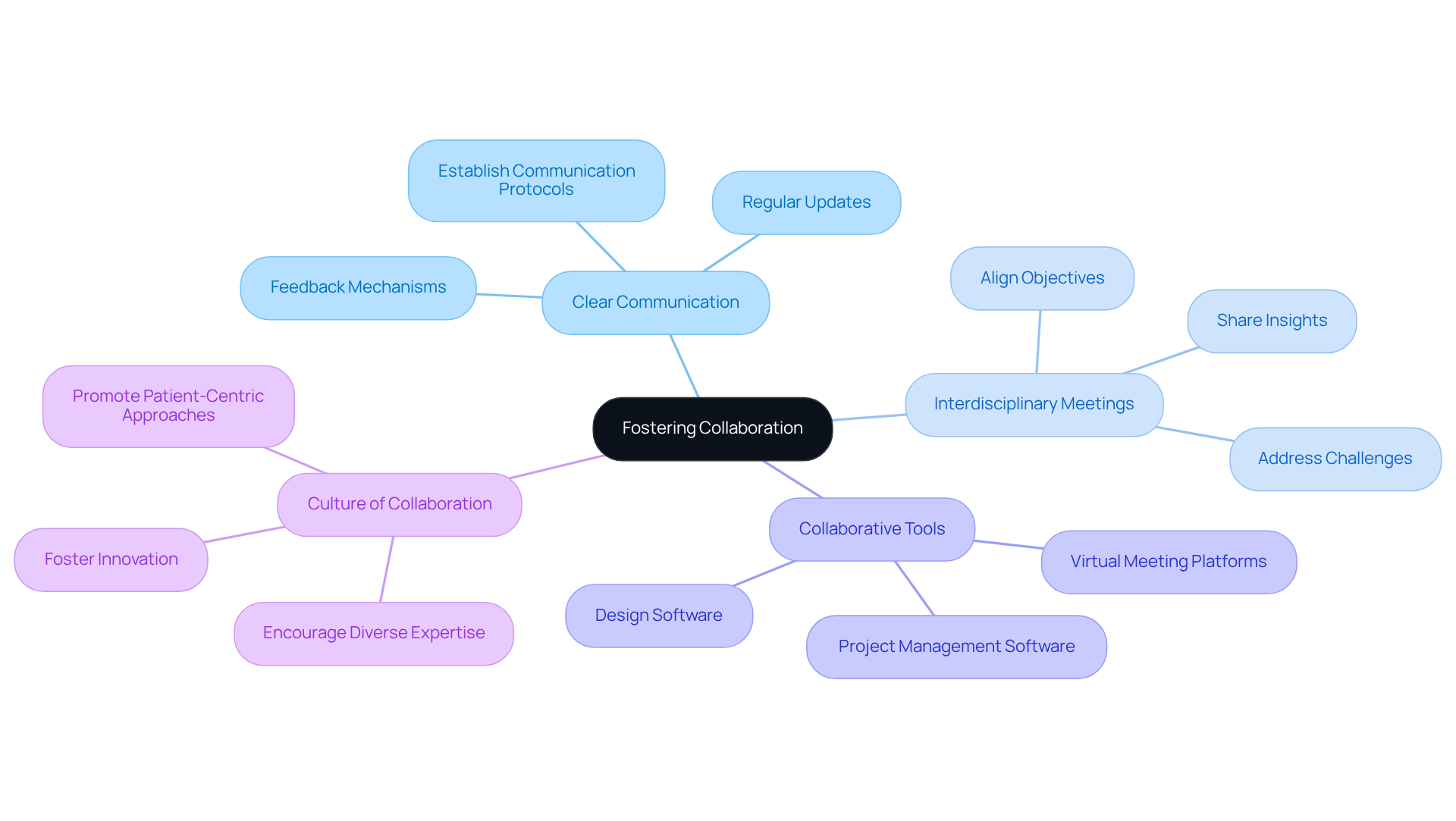
Foster Collaboration Among Engineering Teams and Stakeholders
Teamwork is essential for the success of FPGA projects in the healthcare sector. Clear communication channels among engineering teams, regulatory experts, and stakeholders facilitate the exchange of insights and expedite problem-solving. Regular interdisciplinary meetings align objectives and address potential challenges during the development phase. The use of collaborative tools, such as project management software, increases transparency and optimizes workflows, providing real-time updates and feedback that keep all team members informed and engaged. By fostering a collaborative culture, organizations can promote innovation and ensure that every aspect of the design process is meticulously considered, ultimately resulting in more effective and compliant medical devices.
Conclusion
Field-Programmable Gate Arrays (FPGAs) play a crucial role in the advancement of medical device technology, facilitating real-time processing and tailored solutions that address the specific needs of the healthcare sector. For engineers and developers aiming to foster innovation in medical applications, understanding the significance of FPGAs is essential. This knowledge ensures that devices are not only efficient but also adaptable to the changing requirements of healthcare professionals and patients.
This article outlines four best practices that are vital for effective FPGA engineering in medical devices:
- Acknowledging the importance of regulatory compliance guarantees that designs adhere to the stringent standards established by authorities such as the FDA.
- Utilizing AI-driven engineering enhances design capabilities, leading to improved performance and expedited development cycles.
- Encouraging collaboration among engineering teams and stakeholders fosters transparency and innovation, enabling collective problem-solving.
- Maintaining a thorough understanding of the role of FPGAs empowers teams to develop solutions that significantly enhance the functionality of medical devices.
In a rapidly evolving healthcare landscape, adopting these best practices is imperative for engineers dedicated to improving the quality and safety of medical devices. By emphasizing regulatory compliance, integrating AI technologies, and fostering teamwork, organizations can effectively navigate the complexities of FPGA engineering while contributing to significant advancements in medical technology. The future of healthcare innovation rests with those who comprehend and implement these principles effectively, ultimately resulting in better patient outcomes and more efficient medical solutions.
Frequently Asked Questions
What are FPGAs and their significance in medical devices?
Field-Programmable Gate Arrays (FPGAs) are essential in medical devices as they enable real-time data processing, which is crucial for imaging, diagnostics, and patient monitoring.
How do FPGAs contribute to medical imaging technologies?
FPGAs execute complex algorithms for signal processing in MRI machines, significantly improving image clarity and diagnostic accuracy.
What advantages do FPGAs offer for battery-operated medical devices?
FPGAs are energy-efficient, which extends the operational life of battery-operated devices without compromising performance.
Why is a comprehensive understanding of FPGAs important for engineers and developers in healthcare?
As the healthcare sector focuses on precision and adaptability, understanding FPGAs is vital for fostering innovation in medical device design and development.
How do FPGAs facilitate the design process in medical equipment?
The reconfigurable architecture of FPGAs allows engineers to customize hardware to meet specific requirements, facilitating rapid prototyping and iterative design.