Mastering the Product Manager Requirements Document for Medical Devices
Master the essentials of a product manager requirements document for medical devices.

The landscape of medical device development is evolving rapidly, driven by the increasing demand for innovative, reliable, and compliant solutions that enhance patient care. As organizations strive to navigate the complexities of embedded application development, understanding best practices becomes essential. This article explores key strategies that streamline the development process while ensuring adherence to regulatory standards and quality assurance.
How can teams effectively balance the need for agility and thoroughness in a field where safety is paramount?
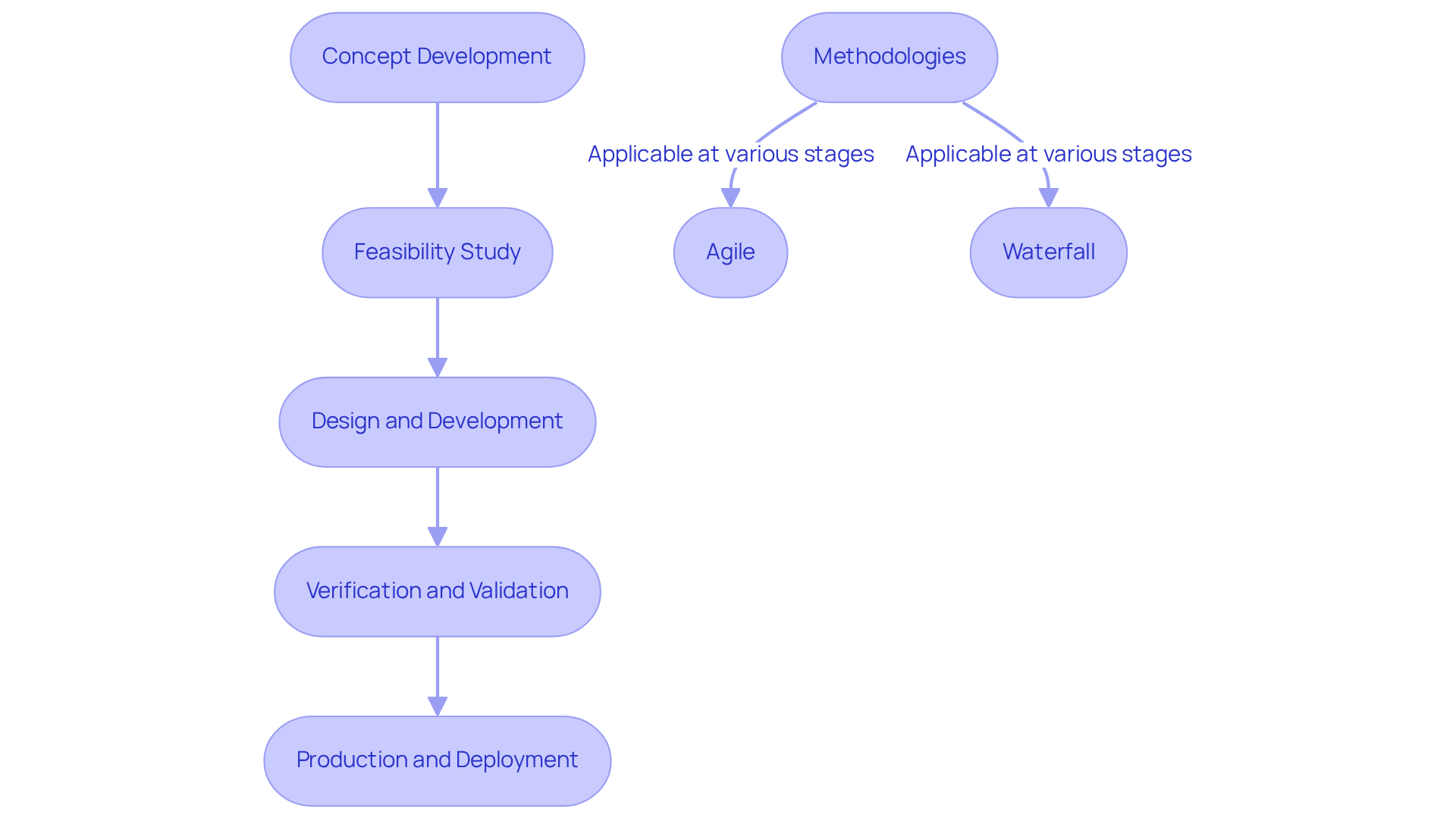
In the development of embedded application development for medical devices, establishing clear phases is essential for success. These stages typically encompass:
Employing methodologies such as Agile or Waterfall can significantly enhance project management effectiveness. Agile promotes iterative development and adaptability, making it particularly advantageous for projects that necessitate frequent adjustments and stakeholder feedback. Conversely, Waterfall offers a structured, linear approach that is beneficial for projects with clearly defined requirements and compliance constraints. For instance, a large hospital network successfully utilized the Waterfall model to digitize patient records, benefiting from its predictable budgeting and compliance alignment.
Statistics reveal that 35% of executive leaders regard organizational agility as a key factor for project success, underscoring the importance of selecting the appropriate methodology based on project complexity and regulatory demands. Understanding these phases and approaches in embedded application development enables teams to optimize processes, mitigate risks, and enhance time-to-market, ultimately resulting in more successful healthcare product creation.
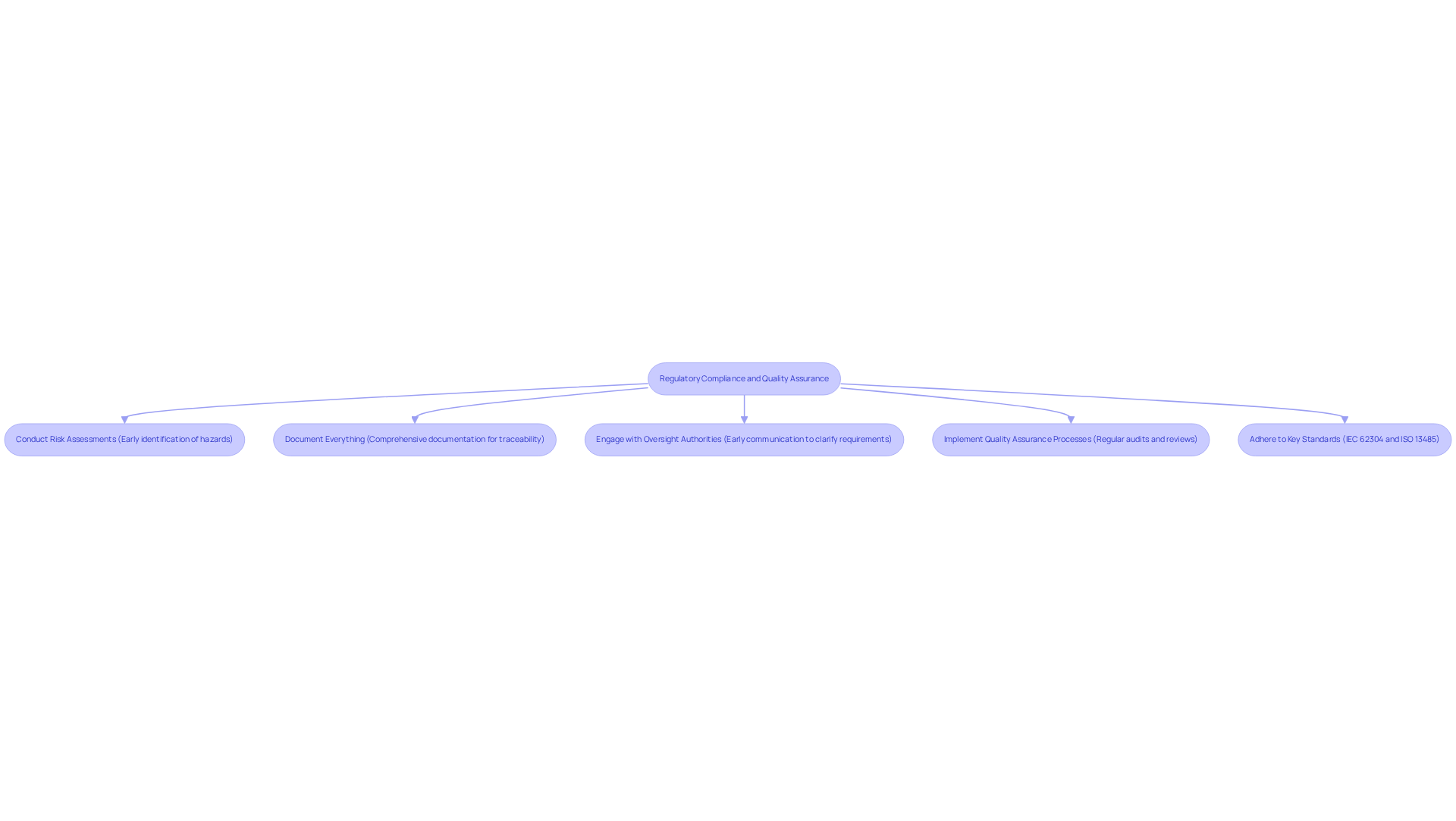
Regulatory compliance is essential in the development of medical devices, underpinned by key standards such as:
To achieve compliance, organizations should adopt the following best practices:
Integrating these practices aligns with the characteristics of successful engineering design projects, such as thorough planning and proactive risk management. A case study highlighting the importance of IEC 62304 compliance illustrates that adherence to this standard enhances patient safety and improves overall software quality. By embedding compliance and quality assurance into the creation process, companies can enhance product safety and efficacy, ultimately leading to improved patient outcomes.
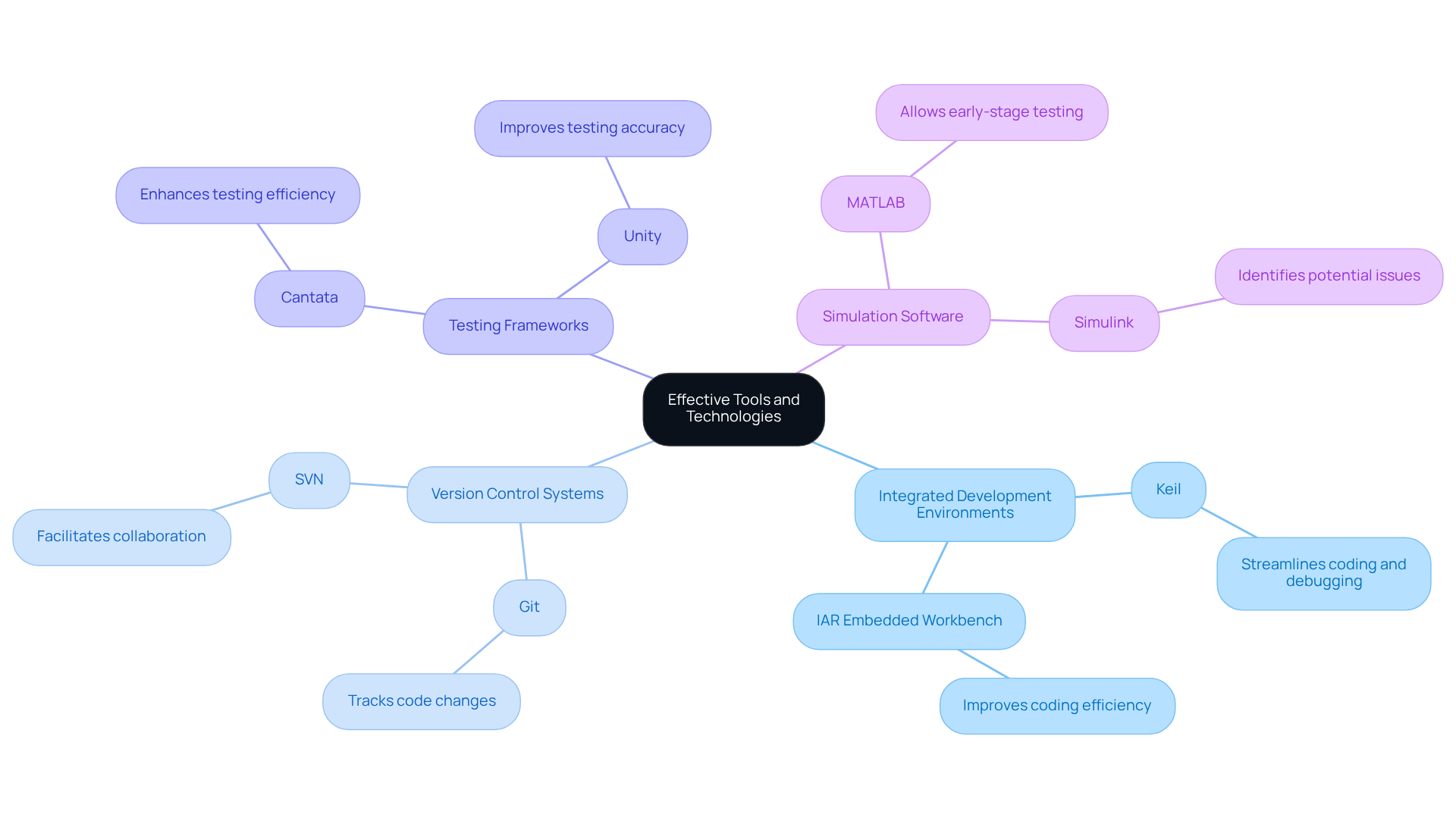
Choosing the appropriate tools and technologies is essential for enhancing the efficiency of embedded application development in the context of medical devices. This focus ultimately leads to projects that are completed on schedule and within budget. Integrated Development Environments (IDEs) play a pivotal role in this process. Tools such as Keil and IAR Embedded Workbench streamline coding and debugging, significantly improving coding efficiency and allowing developers to concentrate on innovation rather than troubleshooting. Effective IDEs can transform the programming landscape, making it crucial for teams to adopt the latest technologies.
Version Control Systems like Git and SVN are indispensable for managing code changes and facilitating collaboration among team members. These systems ensure that all modifications are tracked, thereby reducing the risk of errors and enhancing team productivity.
Testing frameworks, including automated tools like Cantata and Unity, further enhance the development process by improving testing efficiency and accuracy. These frameworks enable thorough validation of software, ensuring that healthcare equipment meets stringent compliance standards and supports successful engineering design projects.
Simulation software, such as MATLAB and Simulink, allows for early-stage testing and validation of designs, enabling teams to identify potential issues before they escalate into costly problems.
When selecting tools, consider the following factors:
By utilizing efficient tools and technologies, such as AI-driven engineering and FPGA design, development teams can enhance productivity, reduce errors, and ensure conformity with industry standards. This approach ultimately leads to the successful introduction of groundbreaking healthcare products.
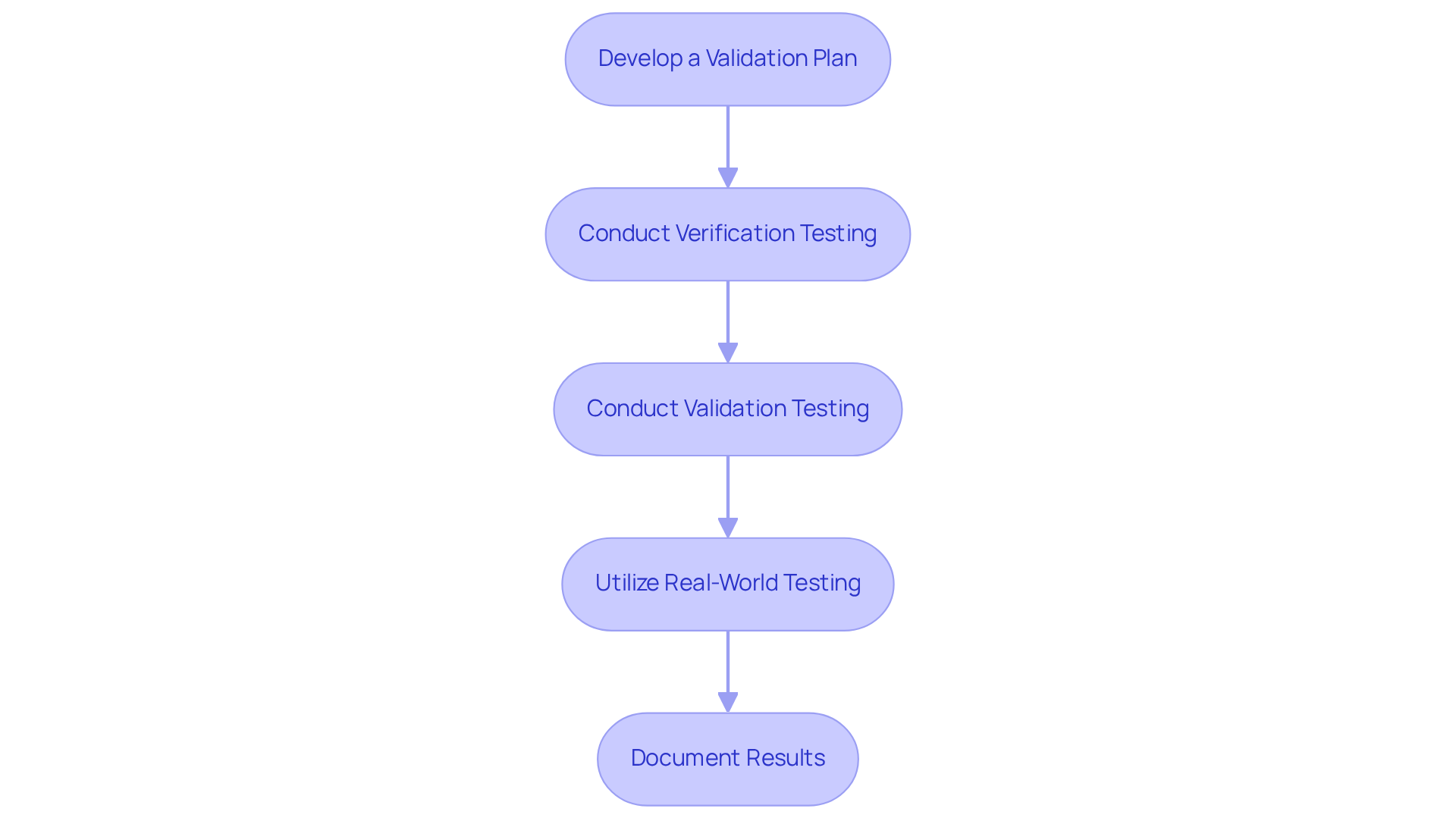
Thorough testing and validation are essential for ensuring the safety and effectiveness of healthcare instruments. Key practices include:
Develop a Validation Plan: Clearly outline the scope, objectives, and methodologies for testing, ensuring alignment with regulatory standards. This organized approach is crucial, as thorough testing is vital for guaranteeing the safety and dependability of healthcare instruments, particularly regarding the documentation compliance assistance provided by Voler Systems.
Conduct Verification Testing: Rigorously test the equipment to confirm it meets design specifications, employing established protocols to ensure reliability. For instance, comprehensive testing not only safeguards patients but also enhances financial success for medical equipment manufacturers by minimizing compliance delays and improving market reputation, which is particularly important for startups navigating the complexities of medical technology.
Conduct Validation Testing: Verify that the equipment fulfills its intended purpose and adheres to compliance standards, which is critical for market readiness. This step is essential, as investing in thorough testing reduces recalls and supports continuous improvement, positioning manufacturers for long-term success in the competitive MedTech landscape, especially with the guidance of Voler Systems.
Utilize Real-World Testing: Engage in clinical trials or user testing to gather feedback and validate performance in practical scenarios, thereby enhancing the device's effectiveness. Industry leaders emphasize that the promise of digital health lies in optimizing care delivery through connected and consistent forums, a process that Voler Systems supports through comprehensive documentation.
Document Results: Maintain meticulous records of all testing activities, results, and corrective actions taken, which is vital for compliance with regulations and quality assurance. This documentation not only facilitates quicker approvals but also fosters trust with stakeholders and end-users, reinforcing the importance of compliance in the development process.
Implementing a robust testing and validation strategy enables organizations to identify potential issues early, ensuring their products are safe, effective, and compliant with regulatory standards. This proactive approach not only enhances product quality but also builds trust with stakeholders and end-users, aligning with Voler Systems' commitment to supporting medical device startups.
The successful development of embedded applications for medical devices relies on a structured approach that integrates best practices across several critical stages. By clearly defining development phases and selecting appropriate methodologies, organizations can streamline their processes, enhance compliance, and ultimately improve patient outcomes. The incorporation of regulatory compliance and quality assurance practices further establishes the foundation necessary for creating safe and effective medical devices.
Key insights from this article underscore the significance of adopting effective tools and technologies, conducting comprehensive testing, and ensuring rigorous validation throughout the development lifecycle. Whether employing Agile or Waterfall methodologies or utilizing advanced Integrated Development Environments and testing frameworks, each element plays a crucial role in mitigating risks and achieving project success. Moreover, thorough documentation and proactive engagement with regulatory authorities are essential for navigating the complex landscape of medical device development.
As the medical technology sector continues to evolve, embracing these best practices will not only enhance the quality and safety of healthcare products but also foster innovation and competitiveness in the market. Stakeholders are encouraged to prioritize these strategies to ensure that their medical devices meet the highest standards of efficacy and compliance, ultimately leading to improved patient care and health outcomes.
What are the main stages of embedded application development for medical devices?
The main stages include Concept Development, Feasibility Study, Design and Development, Verification and Validation, and Production and Deployment.
What is involved in the Concept Development stage?
Concept Development involves identifying the problem and defining the product requirements.
What does the Feasibility Study assess?
The Feasibility Study assesses the technical and financial viability of the project.
What occurs during the Design and Development stage?
During the Design and Development stage, detailed designs and prototypes are created.
How is Verification and Validation conducted?
Verification and Validation ensure that the product meets specifications and compliance requirements.
What is the purpose of the Production and Deployment stage?
The Production and Deployment stage involves transitioning the product to manufacturing and launching it in the market.
What methodologies can be employed in embedded application development?
Methodologies such as Agile and Waterfall can be employed to enhance project management effectiveness.
What are the advantages of using Agile methodology?
Agile promotes iterative development and adaptability, making it beneficial for projects that require frequent adjustments and stakeholder feedback.
When is the Waterfall methodology most beneficial?
The Waterfall methodology is beneficial for projects with clearly defined requirements and compliance constraints, as it offers a structured, linear approach.
Can you provide an example of Waterfall methodology in use?
A large hospital network utilized the Waterfall model to digitize patient records, benefiting from its predictable budgeting and compliance alignment.
What percentage of executive leaders consider organizational agility important for project success?
Statistics reveal that 35% of executive leaders regard organizational agility as a key factor for project success.
Why is it important to understand development phases and methodologies in embedded application development?
Understanding these phases and approaches enables teams to optimize processes, mitigate risks, and enhance time-to-market, leading to more successful healthcare product creation.
