Company: Eye Care Technology Company
Industry: Medical Devices
Use Case: Dry Eye Treatment
Services Used: Electronic Design, Firmware, Diagnostics, Documentation, Verification
Results:
- Transformed prototype into an FDA-approved, market-ready medical device
- Design and verification received first-submission 510(k) approval
- Robust design allowed for high-volume manufacturing
Customer Introduction
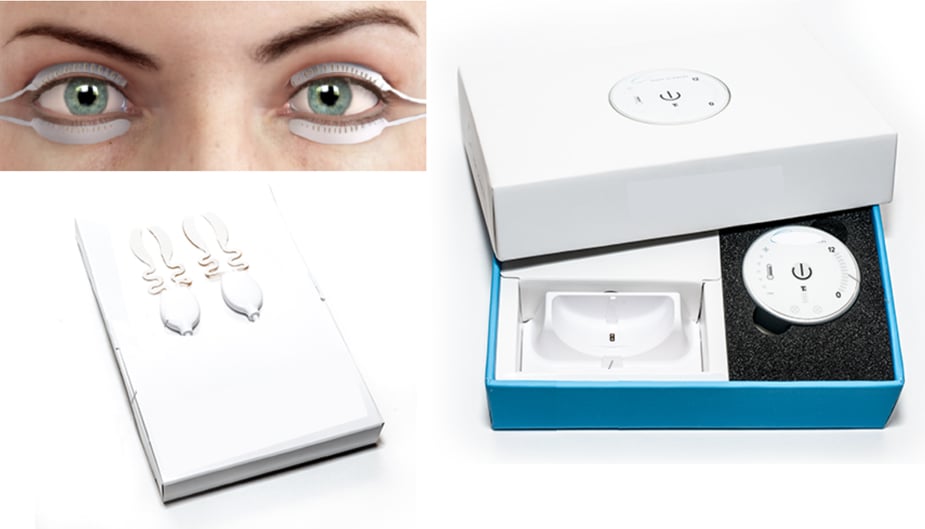
This company produces eye technology for the treatment of eye diseases. It had a new proof-of-concept prototype for dry eye treatment and wanted to market it as a medical device. It needed help doing the design under medical design controls and preparing the device for FDA approval.
Problem/Goal
- Lack of knowledge in designing an electronic medical device.
The company had built a proof-of-concept prototype and needed an electrical engineering expert to create a wearable medical device that applies heat to the eyelids to improve the flow of oil that naturally lubricates the eye.
- Requires 510(k) approval from FDA
The device will meet 510(k) approval from the FDA to be legally marketed in the United States as a medical device treatment for dry eyes and verify proof of its meeting high safety and effectiveness standards.
- Significant manufacturing obstacles
The medical device will be designed for scalability or mass production.
Solution
This company engaged with Voler Systems because of its extensive experience in wearable medical device design and development and knowledge of strict federal regulatory requirements.
Voler Systems’ engineers identified what was needed to take the device from a proof-of-concept to reality. They implemented a development process to ready it for submission to the FDA. This systematic approach resulted in Voler Systems delivering the following solutions:
- Redesigned device electronics
- Created firmware to operate the electronics
- Created a risk analysis in conjunction with the company
- Created and executed the verification protocol
- Created document package for software and verification results and placed in the company’s quality system for submission to FDA
Results & Benefits
- Successful redesign gained approval from FDA as a wearable medical device, confirming its safety and efficacy for patient use.
- Design approved by FDA on the first submission of 510(k), saving approximately six months in development time and costs.
- Robust medical device design allowed for high-volume manufacturing, enabling the client to meet market demand effectively.
- Innovative wearable medical device design and functionality improved the quality of life for millions suffering from dry eyes.
Inspiration
Voler Systems’ technical expertise, comprehensive regulatory knowledge, and commitment to high-quality standards enable the successful transformation of innovative prototypes into market-ready medical devices, attesting to our ability to deliver effective solutions.
“Partnering with Voler Systems quickly transformed our ambitious vision of a dry-eye-relief wearable from a prototype into an FDA-approved, market-ready medical device. Their invaluable expertise and belief in our vision led to our device’s successful launch and real-life impact, offering relief to countless users.”
Contact Voler Systems today and let us help you transform your vision into an impactful, market-ready medical device.

