4 Best Practices for Effective PCB Design Board in Medical Devices
Discover best practices for effective PCB design board in medical devices to ensure...

Ensuring the safety and reliability of medical devices is paramount, especially as technology evolves rapidly. The IEC 61508 standard provides a crucial framework for manufacturers, guiding them through the complexities of functional safety in electrical and electronic systems. By exploring the principles of this standard, readers will discover essential strategies for compliance that not only safeguard patient health but also enhance the overall quality of medical devices. However, given the numerous challenges associated with implementing these standards, how can manufacturers effectively navigate the regulatory landscape to achieve compliance and ensure safety?
IEC is a vital global standard that establishes a framework for ensuring the functional security of electrical, electronic, and programmable electronic systems, particularly within the medical device sector. Compliance with IEC standards is critical for mitigating risks associated with device failures that could jeopardize patient safety. The standard promotes a lifecycle approach, highlighting the significance of risk assessment, design, implementation, and continuous maintenance of safety-related systems. For manufacturers, understanding this standard is essential to meet regulatory requirements and uphold high quality standards throughout the operational lifespan of their products.
Key aspects of IEC 61508 include:
By thoroughly understanding these principles, manufacturers can adeptly navigate the complexities of regulations, thereby enhancing the safety and efficacy of their medical devices. Real-world applications of the IEC 61508 functional safety standard, such as in infusion pumps, illustrate its effectiveness in addressing hazards like incorrect flow rates, reinforcing the standard's crucial role in protecting patient health.
To achieve compliance with IEC 61508, manufacturers should follow these essential steps:
Conduct a Risk Assessment: Identify potential hazards associated with the medical device and evaluate the risks. This involves determining the necessary Integrity Level (SIL) for each protective function based on the severity of possible harm. Effective risk evaluation is crucial, as it lays the foundation for all subsequent precautionary measures.
Define Protection Criteria: Establish clear protection requirements derived from the risk assessment. This includes detailing the necessary performance levels for protective functions, ensuring that all critical aspects are comprehensively addressed.
Design for Protection: Implement design practices that incorporate protective features. This may include redundancy, fail-safe mechanisms, and robust testing protocols to ensure reliability. Prioritizing protection in design not only meets regulatory requirements but also enhances overall product quality. Voler Systems emphasizes the integration of innovative design practices to bolster security and compliance in medical technology development.
Develop a Risk Management Plan: Create a comprehensive plan that outlines how risk will be managed throughout the device's lifecycle. This should encompass procedures for verification, validation, and change management, ensuring that security remains a priority at every stage.
Documentation: Maintain thorough documentation of all processes, decisions, and changes made during the development and lifecycle of the device. This is essential for demonstrating adherence during audits and for ongoing risk assessments. Voler Systems provides vital documentation support services, assisting startups in navigating the complexities of regulatory requirements.
Training and Competence: Ensure that all personnel involved in the design and development process are adequately trained in IEC standards and risk management practices. Ongoing education fosters a culture of security and compliance with IEC 61508 functional safety within the organization.
By systematically executing these steps, manufacturers can align their processes with IEC 61508 functional safety standards, thereby enhancing the protection and reliability of their medical devices while effectively navigating the evolving landscape of regulatory requirements, supported by Voler Systems' expertise in compliant and reliable electronic device design.
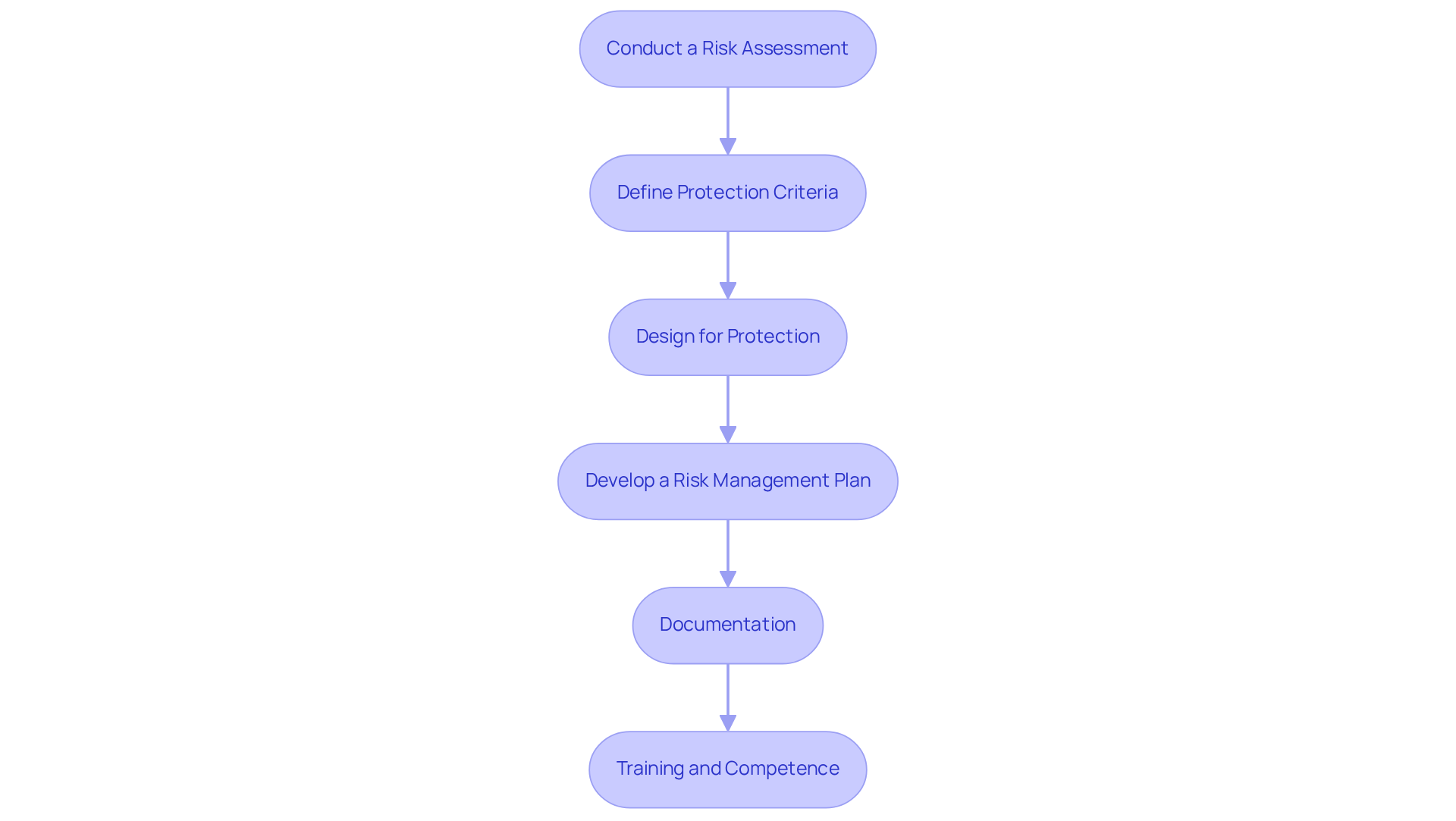
Effective documentation and validation are essential for demonstrating compliance with IEC 61508 functional safety standards. The following key steps outline the process:
Create a Compliance File: Compile all documentation related to the risk management plan, risk assessments, design specifications, and testing protocols. This file must be comprehensive and organized for easy access during audits, ensuring that all necessary information is readily available.
Validation Activities: Conduct thorough validation tasks to ensure that the device meets specified protection criteria. This includes:
Change Control Procedures: Implement robust change control procedures to manage modifications to the device or its documentation. This guarantees that any modifications are evaluated for their impact on security and regulations, preserving the integrity of the safety management system.
Audit Trails: Maintain detailed records of all compliance-related activities, including audits, reviews, and corrective actions taken. This provides a clear trail of accountability and supports regulatory inspections, demonstrating a commitment to safety and reliability.
Regular Updates: Ensure that adherence documentation remains current with any changes in regulations or standards. This guarantees continuous compliance with IEC 61508 functional safety standards and reflects the current condition of the device, which is essential for preserving market access and stakeholder confidence.
By following these steps, manufacturers can effectively document and confirm their adherence efforts, providing assurance to regulators and stakeholders regarding the reliability of their medical devices. Compliance with IEC standards not only enhances safety but also mitigates risks associated with system failures, ultimately safeguarding lives and minimizing economic impacts.
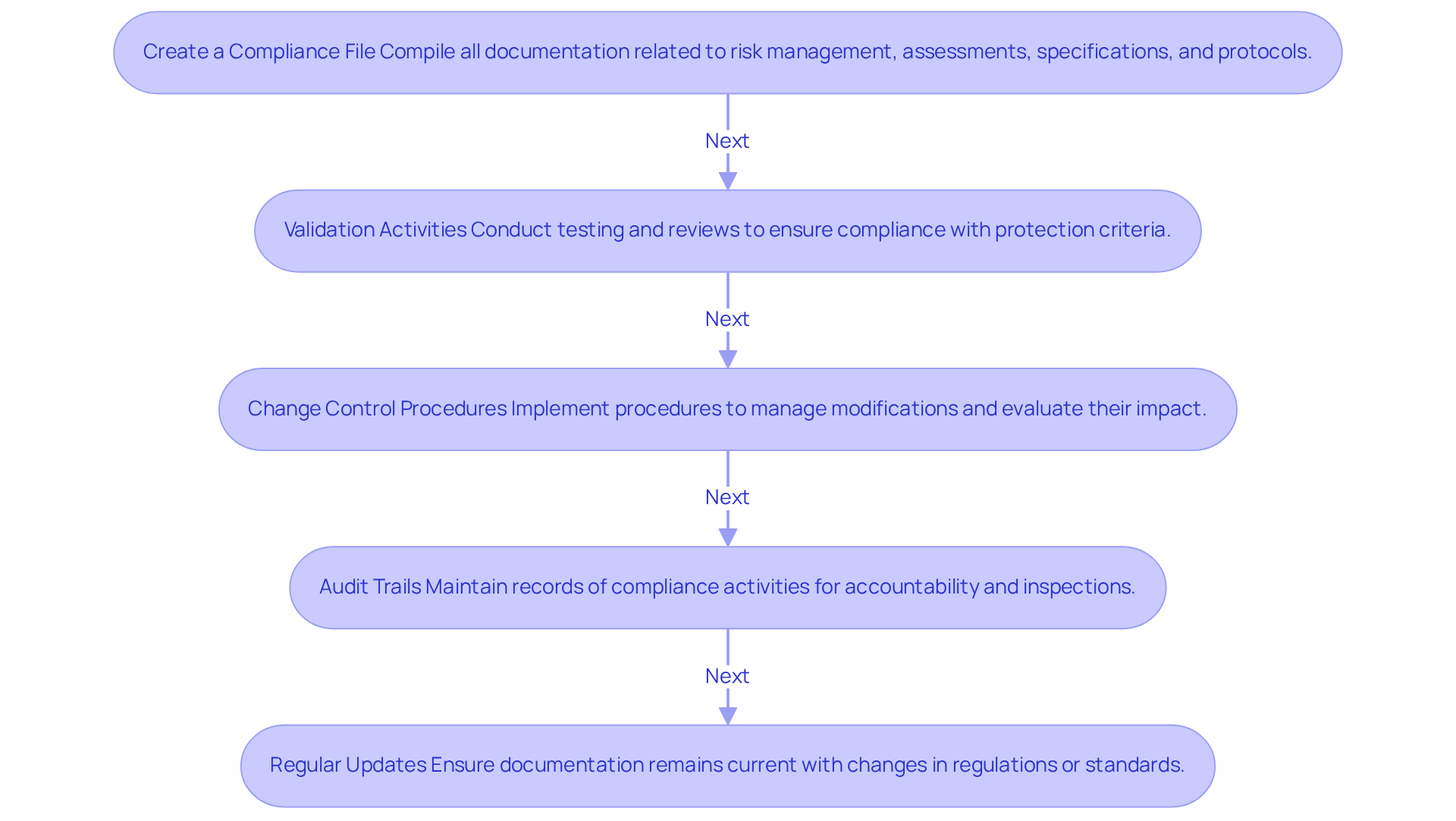
Producers frequently face significant challenges in achieving IEC standards. Below are common issues along with effective strategies to navigate them:
Understanding the Standard: The complexity of IEC standards can be daunting. To address this:
Integration into Development Processes: Incorporating safety requirements into existing workflows can be challenging. Consider these solutions:
Resource Allocation: Limited resources can impede adherence efforts. To optimize resource utilization:
Documentation Gaps: Inadequate documentation can lead to regulatory failures. To mitigate this risk:
Keeping Up with Changes: The regulatory landscape is continually evolving. To stay informed:
By proactively addressing these challenges, manufacturers can enhance their compliance efforts, ensuring the safety and effectiveness of their medical devices.
Understanding and implementing IEC 61508 is essential for ensuring the functional safety of medical devices. This standard serves as a framework for mitigating risks associated with device failures and emphasizes a comprehensive lifecycle approach to safety management. By prioritizing compliance with IEC 61508, manufacturers can enhance the reliability of their products and safeguard patient health.
The article outlines critical steps for achieving compliance, including:
It highlights the importance of ongoing training and proactive troubleshooting to navigate common challenges faced by manufacturers. By systematically addressing these key components, organizations can align their processes with IEC standards and reinforce the safety and efficacy of their medical devices.
Ultimately, adherence to IEC 61508 is not merely a regulatory obligation; it represents a commitment to patient safety and quality assurance in medical technology. Manufacturers are encouraged to embrace these guidelines, invest in training, and stay informed about evolving standards to ensure that their devices not only meet compliance requirements but also contribute positively to healthcare outcomes.
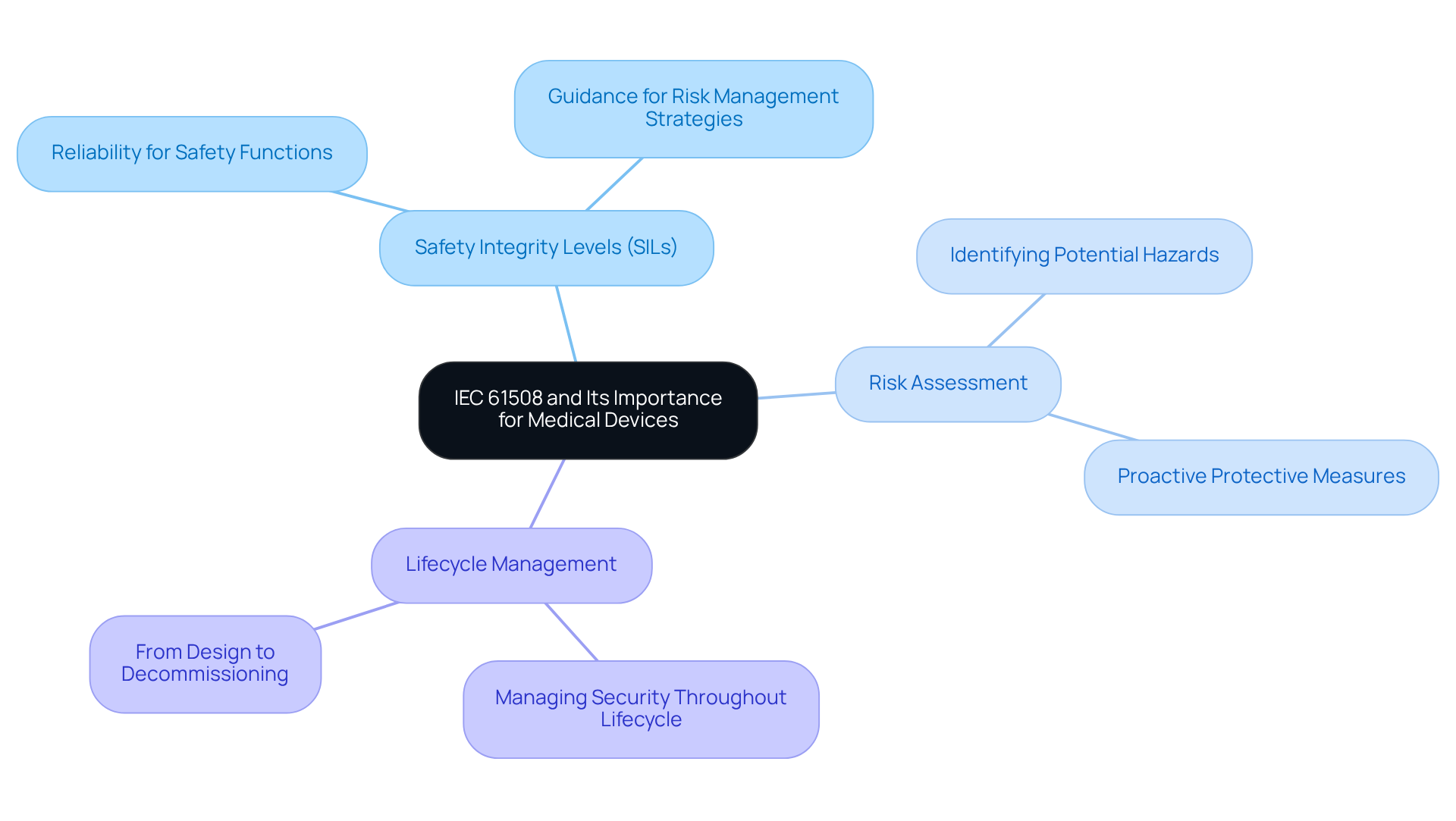
What is IEC 61508?
IEC 61508 is a global standard that provides a framework for ensuring the functional security of electrical, electronic, and programmable electronic systems, particularly in the medical device sector.
Why is compliance with IEC 61508 important for medical devices?
Compliance with IEC 61508 is critical for mitigating risks associated with device failures that could jeopardize patient safety, ensuring high quality standards throughout the operational lifespan of medical devices.
What are Safety Integrity Levels (SILs)?
Safety Integrity Levels (SILs) are levels that delineate the reliability necessary for safety functions, guiding manufacturers in effective risk management strategies.
How does risk assessment relate to IEC 61508?
Risk assessment is a systematic method for identifying and mitigating potential hazards linked to device failures, ensuring proactive protective measures are established in accordance with IEC 61508.
What is lifecycle management in the context of IEC 61508?
Lifecycle management refers to the importance of managing security throughout the entire lifecycle of a device, from initial design to decommissioning, as emphasized by IEC 61508.
How does understanding IEC 61508 benefit manufacturers?
By understanding IEC 61508, manufacturers can navigate regulatory complexities, enhance the safety and efficacy of their medical devices, and meet regulatory requirements.
Can you provide an example of IEC 61508 in practice?
Real-world applications of IEC 61508, such as in infusion pumps, demonstrate its effectiveness in addressing hazards like incorrect flow rates, reinforcing the standard's role in protecting patient health.
