Master Machine Communication: Best Practices for Medical Devices
Explore best practices for effective machine communication in medical devices for...

Creating a Product Requirements Document (PRD) is crucial for aligning teams and ensuring successful product development, especially in complex sectors such as medical devices. This guide explores the essential components and steps required to develop an effective PRD, emphasizing the importance of clarity and collaboration throughout the process.
Nonetheless, many teams encounter common pitfalls that can hinder their progress. How can organizations effectively navigate these challenges to produce a document that genuinely fosters innovation and addresses user needs?
A Product Requirements Document (PRD) serves as a crucial tool that delineates the key features, functionalities, and constraints of a product. It ensures alignment among all stakeholders - managers, engineers, and designers - regarding the goals and significance of the offering. Acting as a single source of truth throughout the development lifecycle, the PRD mitigates misunderstandings and curtails scope creep. By clearly articulating the specific issue the offering addresses, the PRD enables teams to make informed decisions and prioritize features effectively.
This organized approach not only enhances collaboration but also significantly increases the likelihood of a successful launch. Companies that employ well-crafted PRDs report a higher success rate in innovation, with top performers achieving a 76% success rate in bringing new offerings to market. Furthermore, effective PRDs have been shown to reduce new product development (NPD) failure rates, which can soar to 90% for startups, by fostering a shared understanding of objectives and user requirements among all team members.
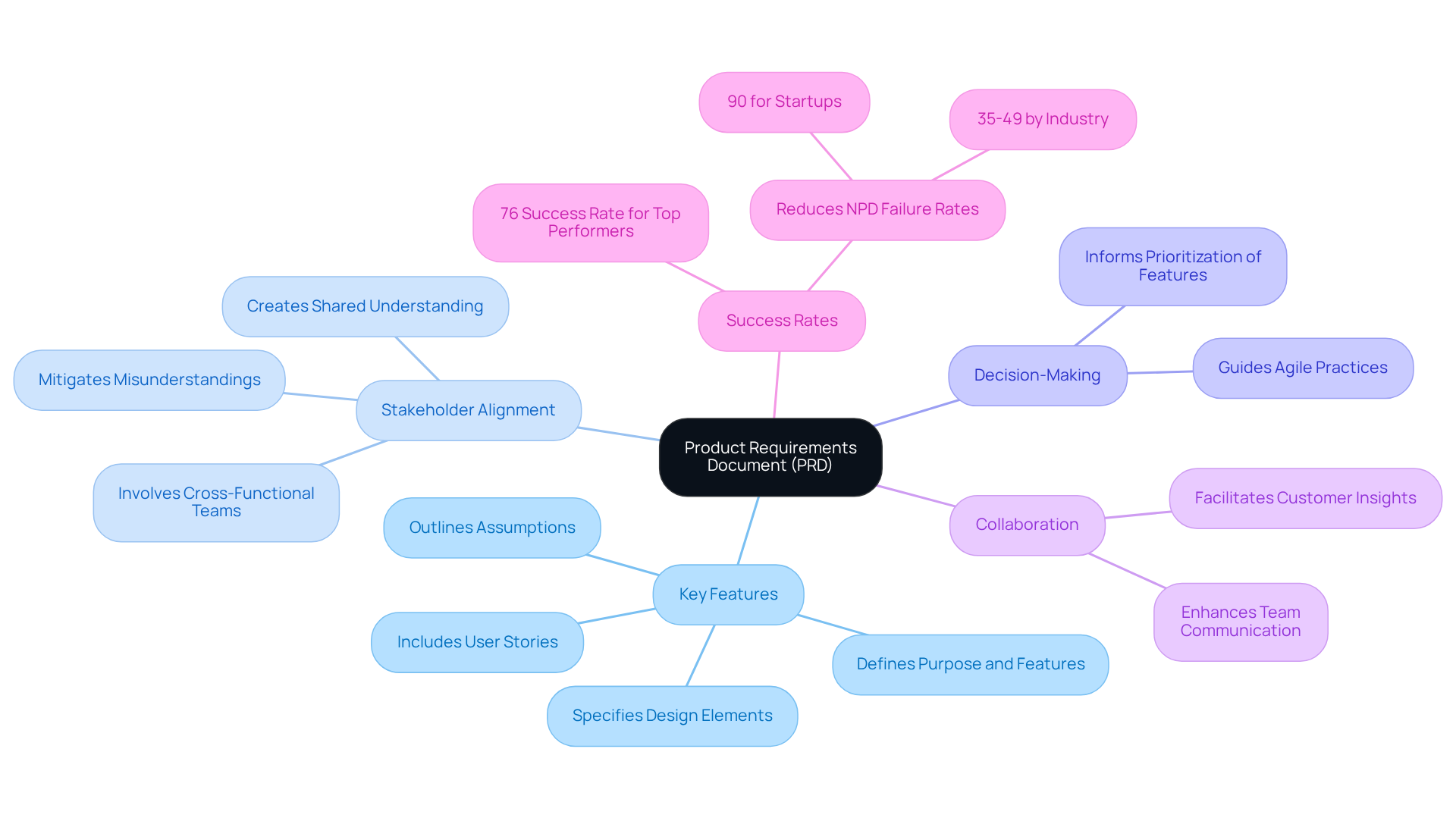
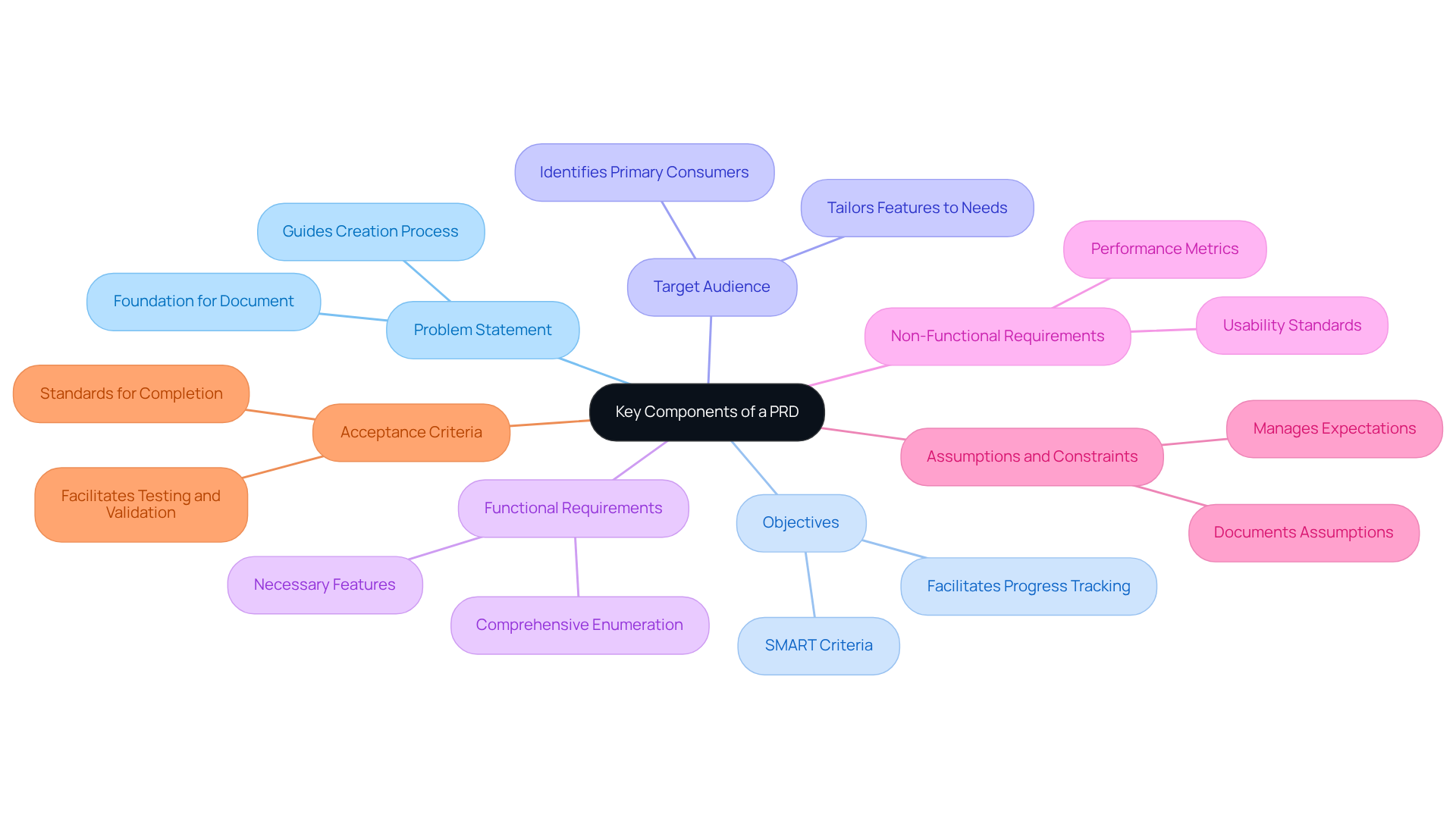
A well-organized product requirement document example is crucial for the successful development of medical devices, particularly in the rapidly evolving landscape of wearable and IoT solutions. It typically encompasses the following key components:
Problem Statement: Clearly articulate the specific issue the solution aims to address. This statement serves as the foundation for the entire document, guiding the creation process and ensuring alignment with user needs.
Objectives: Define the item's goals using the SMART criteria - specific, measurable, achievable, relevant, and time-bound. This clarity facilitates progress tracking and success assessment, especially when utilizing Voler Systems' expert electronic design services to expedite development.
Target Audience: Identify the primary consumers of the item and their unique requirements. Understanding the audience is essential for tailoring features that enhance experience and satisfaction, particularly for medical devices that must comply with regulatory standards.
Functional Requirements: Enumerate the necessary features and functionalities that the item must possess to effectively meet user needs. This section should be comprehensive to prevent ambiguity during the process, ensuring that the outcome aligns with Voler Systems' innovative embedded systems design strategies.
Non-Functional Requirements: Specify performance metrics, usability standards, and compliance with regulatory requirements. These criteria ensure that the item not only meets functional expectations but also adheres to industry standards, which is vital for medical devices developed by Voler Systems, including wearable devices and heart pumps.
Assumptions and Constraints: Document any assumptions made during the development process and constraints that may affect the project. This transparency aids in managing expectations and prepares the team for potential challenges, particularly when navigating compliance from prototype to production.
Acceptance Criteria: Define the standards that must be met for the item to be considered complete and ready for launch. Clear acceptance criteria facilitate effective testing and validation, ensuring the item fulfills its intended purpose.
Industry leaders emphasize that a well-articulated problem statement is essential for guiding development and aligning team efforts. Research indicates that a product requirement document example with clearly defined objectives and client needs significantly enhances project success rates, making it indispensable in the medical device sector, especially when supported by Voler Systems' AI-assisted engineering and ultra-low power design strategies.
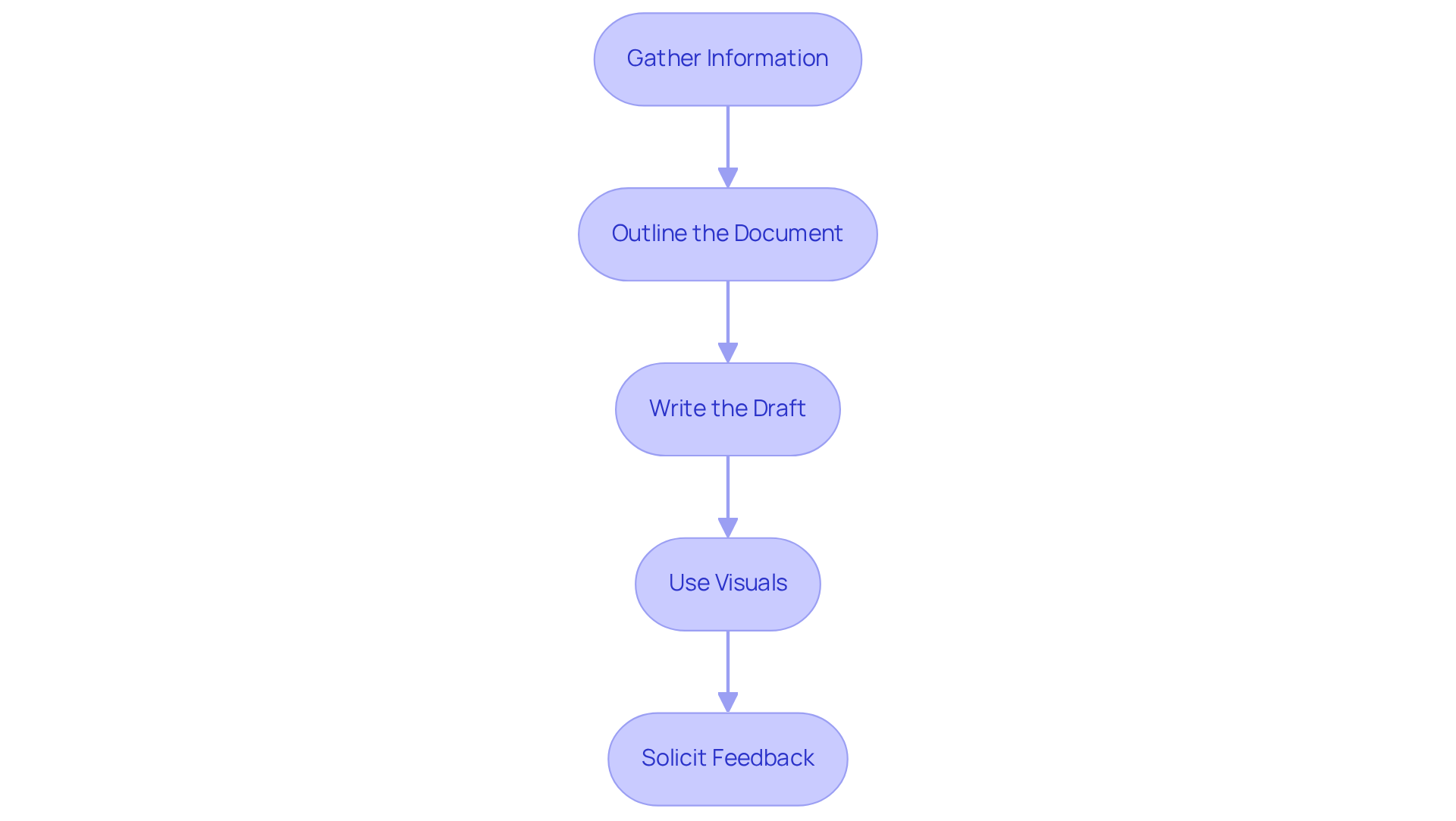
To draft your PRD effectively, follow these essential steps:
Gather Information: Engage with stakeholders, including product owners, designers, and engineers, to collect comprehensive details on customer needs, market research, and technical constraints. This collaborative effort ensures diverse perspectives are considered, enhancing the relevance of the product requirement document example.
Outline the Document: Create a structured outline that includes key components such as goals, assumptions, user stories, and design elements. This roadmap will guide your writing and help maintain clarity throughout the product requirement document example.
Write the Draft: Populate each section of the outline with detailed information, using clear and concise language. Aim for a balance between technical detail and accessibility in the product requirement document example to ensure that all stakeholders can easily understand the content.
Use Visuals: Integrate diagrams, charts, or mockups to provide visual context for complex ideas. Research indicates that visuals can enhance understanding and retention by up to 78%. Furthermore, individuals retain 65% of information three days after viewing an image with data, compared to just 10% from auditory information. This approach enhances the effectiveness and engagement of your product requirement document example.
Solicit Feedback: Share the draft with key stakeholders to gather their input and suggestions. This collaborative approach not only identifies gaps or misunderstandings early in the process but also fosters a sense of ownership among team members, leading to a more robust final document. Additionally, consider using tools like Confluence to facilitate collaboration and streamline the PRD creation process.
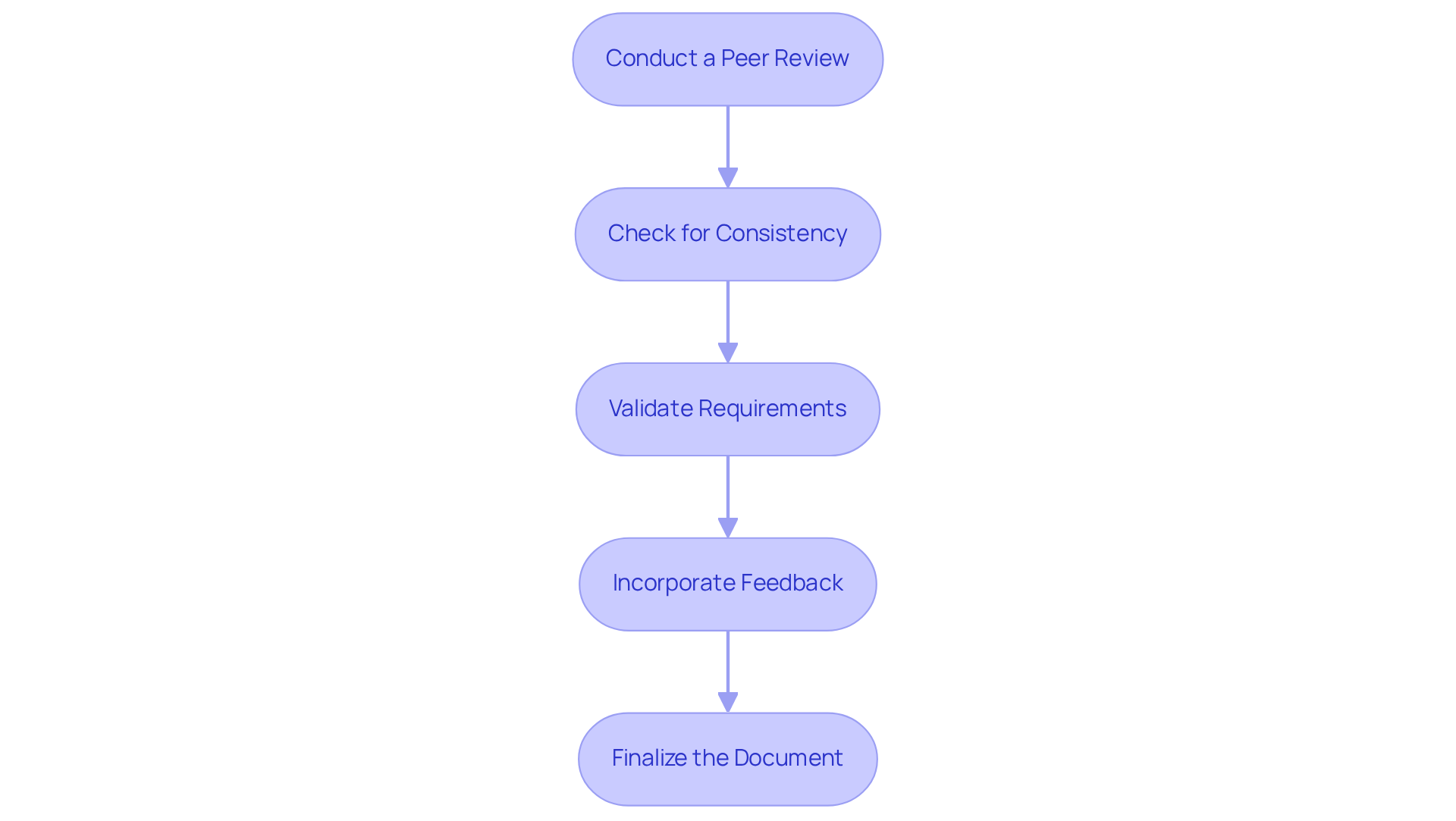
To ensure your Product Requirements Document (PRD) is effective and meets industry standards, particularly in the medical device sector, follow these essential steps for review and refinement:
Conduct a Peer Review: Engage team members to evaluate the document for clarity, completeness, and accuracy. Constructive feedback is vital for identifying areas that may need improvement. The Worldcom Peer Review process exemplifies high standards in peer evaluations, ensuring thorough assessments that enhance document quality. This practice aligns with Voler Systems' commitment to compliance support for startups in medical technology.
Check for Consistency: Maintain uniformity in terminology and formatting throughout the document. Consistency enhances professionalism and improves readability, which is crucial in technical documentation. Research indicates that consistent documentation practices lead to better understanding and fewer errors in implementation, a principle that Voler Systems emphasizes in their engineering design projects.
Validate Requirements: Cross-reference the requirements with client needs and business objectives. This alignment is essential to ensure that the PRD accurately reflects the goals of the project and meets stakeholder expectations. For example, the Airbnb Total Price PRD is a product requirement document example that illustrates how aligning requirements with user needs can result in successful outcomes, a strategy that Voler Systems employs in navigating compliance in wearable medical device creation.
Incorporate Feedback: Revise the document based on the feedback received. This may involve clarifying language, adding necessary details, or eliminating superfluous information to streamline the content. Engaging in this iterative process is crucial for refining the PRD, mirroring the essential actions required during highly successful engineering design projects that Voler Systems advocates.
Finalize the Document: After implementing all revisions, prepare the final version of the PRD for distribution to stakeholders. A well-organized and refined document is essential for enabling effective communication and project success, ensuring that your offering aligns with the innovative medical technology standards that Voler Systems supports.
By following these steps, you can improve the quality and effectiveness of your PRD, ensuring it serves as a dependable basis for creating in the medical device sector.
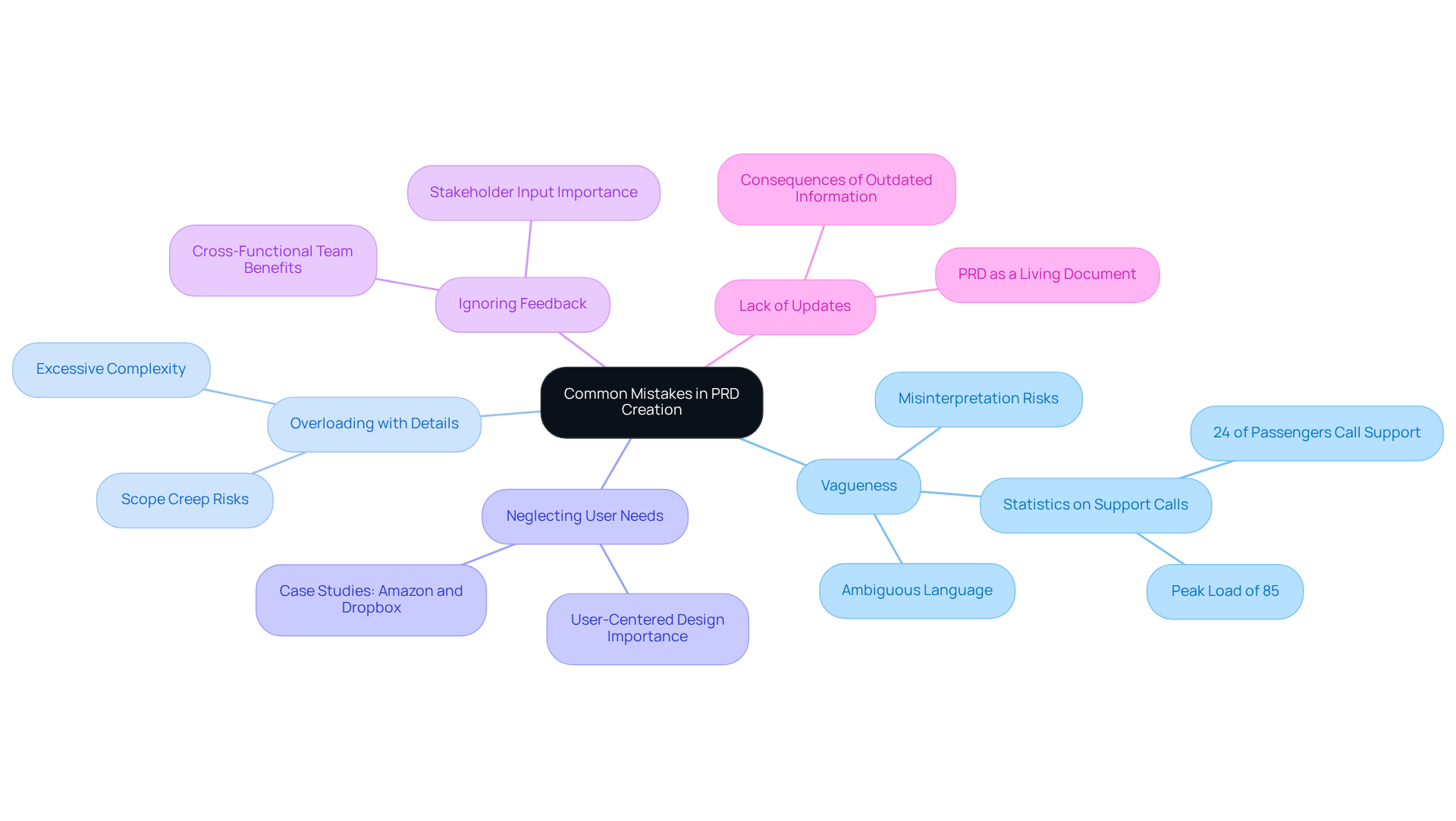
Creating an effective Product Requirements Document (PRD) requires vigilance against several common pitfalls:
Vagueness: Ambiguous language can lead to misinterpretation and confusion among development teams. Clearly defining requirements and expectations is essential to ensure alignment. For instance, statistics indicate that 24% of passengers whose messages fail to deliver call support, resulting in peak loads of 85%. This underscores the importance of clear communication in specifications.
Overloading with Details: While comprehensive information is valuable, excessive detail can overwhelm readers. It is crucial to prioritize essential requirements and eliminate unnecessary complexity to maintain focus.
Neglecting User Needs: Keeping the end-user in mind is vital. Disregarding consumer needs can result in an offering that fails to meet market demands, ultimately impacting its success. Effective product requirement document examples from companies like Amazon and Dropbox demonstrate clarity and specificity, ensuring that all stakeholders understand the goals and functionalities of the offering.
Ignoring Feedback: Stakeholder input is critical for identifying potential issues. Fostering collaboration and integrating diverse perspectives into the PRD enhances its effectiveness. Involving cross-functional teams can significantly improve the understanding of customer needs and validate solutions.
Lack of Updates: The PRD should be treated as a living document that evolves with the project. Frequent updates are essential to reflect changes in requirements, user feedback, or market conditions, ensuring its continued relevance throughout the development process. This practice is crucial for maintaining alignment with the latest offerings and requirements.
By avoiding vagueness and concentrating on user-centered requirements, teams can significantly enhance their development outcomes. Additionally, defining clear success metrics in the PRD establishes a target for the team and facilitates effective evaluation of the product post-launch.
A well-structured Product Requirements Document (PRD) is essential for ensuring clarity and alignment among stakeholders throughout the product development process. By establishing a comprehensive framework that outlines objectives, user needs, and key functionalities, a PRD serves as a vital reference point that minimizes misunderstandings and enhances collaboration. Ultimately, a well-crafted PRD not only streamlines development but also significantly increases the likelihood of a successful product launch.
The article emphasizes several critical components of an effective PRD, including:
It highlights the importance of gathering diverse perspectives during the drafting process and the necessity of iterative reviews to refine the document. By avoiding common pitfalls such as vagueness and neglecting user needs, teams can create a PRD that is both actionable and aligned with market demands.
In conclusion, investing time and effort into creating a thorough and precise PRD can substantially impact the success of a product, particularly in complex fields like medical devices. As organizations strive for innovation and efficiency, adopting best practices in PRD development is not just beneficial; it is essential. Embrace these guidelines to enhance your product development efforts and ensure that your offerings meet both stakeholder expectations and user needs effectively.
What is the purpose of a Product Requirements Document (PRD)?
A PRD serves as a crucial tool that outlines the key features, functionalities, and constraints of a product, ensuring alignment among stakeholders and acting as a single source of truth throughout the development lifecycle.
How does a PRD contribute to the success of a product launch?
By clearly articulating the specific issue the product addresses, a PRD enhances collaboration, mitigates misunderstandings, and reduces scope creep, significantly increasing the likelihood of a successful launch.
What are the benefits of using a well-crafted PRD?
Companies that utilize well-crafted PRDs report a higher success rate in innovation, with top performers achieving a 76% success rate in bringing new offerings to market, and they also experience reduced failure rates in new product development.
What are the key components of a well-organized PRD?
The key components of a PRD include:
Why is a clear problem statement important in a PRD?
A clear problem statement serves as the foundation for the entire document, guiding the creation process and ensuring alignment with user needs, which is essential for project success.
What does the objectives section of a PRD entail?
The objectives section defines the item's goals using the SMART criteria: specific, measurable, achievable, relevant, and time-bound, facilitating progress tracking and success assessment.
How does understanding the target audience benefit the development process?
Identifying the primary consumers and their unique requirements helps tailor features that enhance user experience and satisfaction, especially for products that must comply with regulatory standards.
What are functional and non-functional requirements in a PRD?
Functional requirements enumerate the necessary features and functionalities the product must possess, while non-functional requirements specify performance metrics, usability standards, and compliance with regulatory requirements.
What should be included in the assumptions and constraints section of a PRD?
This section should document any assumptions made during development and constraints that may affect the project, aiding in managing expectations and preparing the team for potential challenges.
What are acceptance criteria in a PRD?
Acceptance criteria define the standards that must be met for the product to be considered complete and ready for launch, facilitating effective testing and validation.
