Master Machine Communication: Best Practices for Medical Devices
Explore best practices for effective machine communication in medical devices for...

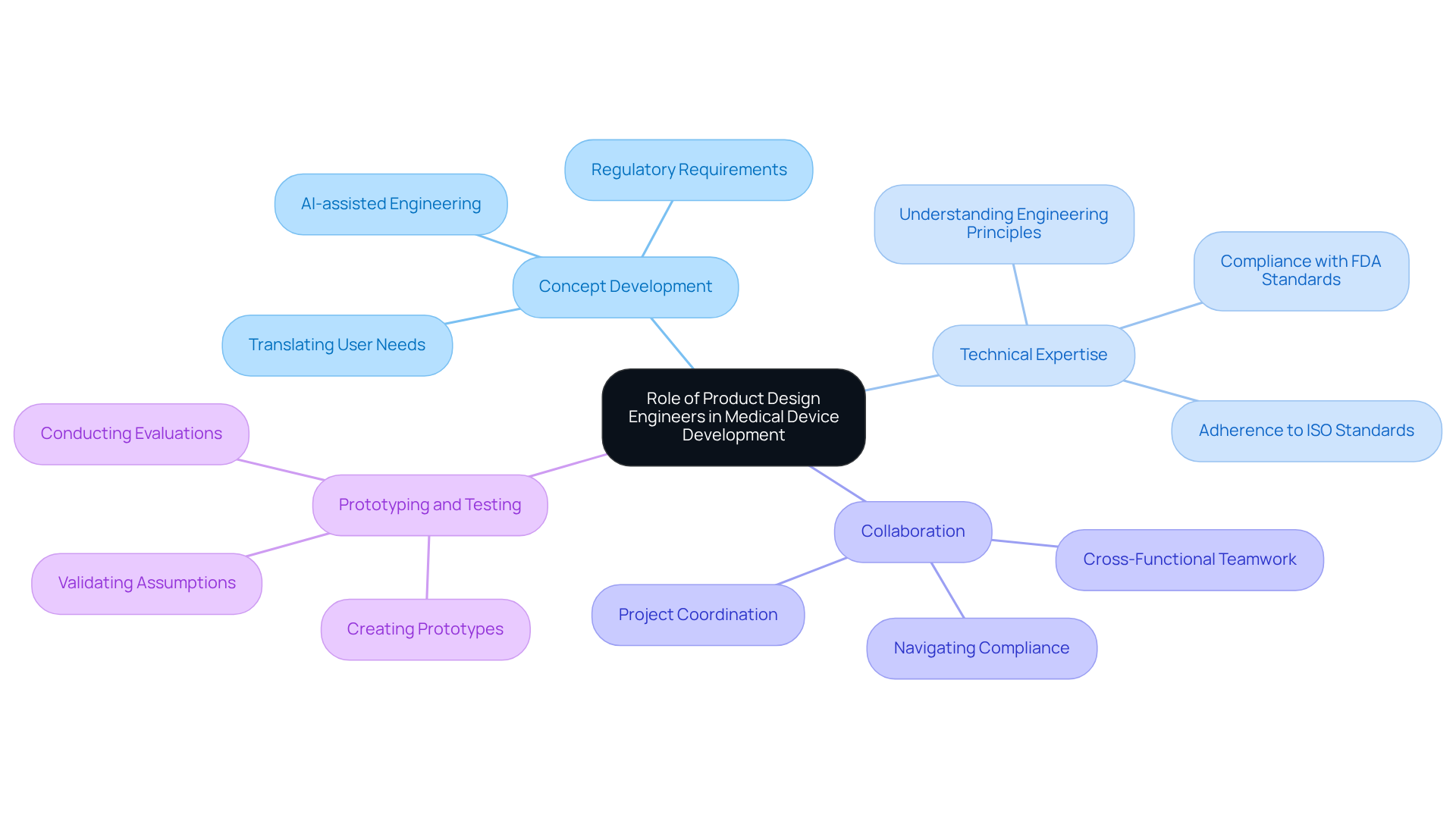
In the rapidly evolving landscape of medical device development, product design engineers play an increasingly pivotal role. These professionals bridge the gap between innovative ideas and practical applications, ensuring that products not only meet stringent regulatory standards but also satisfy market demands. By exploring best practices for collaboration with product design engineering companies, organizations can enhance their potential for innovation and compliance.
However, how can teams effectively engage these experts from the outset to navigate the complexities of the development process and achieve optimal outcomes?
Product development engineers play a vital role in the success of medical devices, acting as a bridge between innovative concepts and practical applications. Their primary responsibilities include:
The impact of engineers on the success rates of healthcare instruments is significant; initiatives that incorporate their expertise from the outset typically achieve higher compliance and market readiness. For instance, successful healthcare equipment development projects have demonstrated that early integration of engineering insights can lead to a 90% success rate in delivering products on time and within budget. The global healthcare equipment creation and development services market was projected to reach approximately USD 12.79 billion by 2025, with an expected CAGR of 14.42% from 2026 to 2033, highlighting the growing need for skilled engineers in this field. Industry leaders emphasize that the involvement of design engineers is not merely beneficial but essential for managing the complexities of healthcare equipment development in 2026 and beyond. As Mariam Faizullabhoy observes, 'The designing & engineering segment accounted for the largest market revenue share of 37.4% in 2023 and is projected to grow at a 12.4% CAGR, as it is crucial for innovation and compliance in medical device development.' Their contributions are vital in fostering innovation, enhancing quality, and ultimately improving patient outcomes.
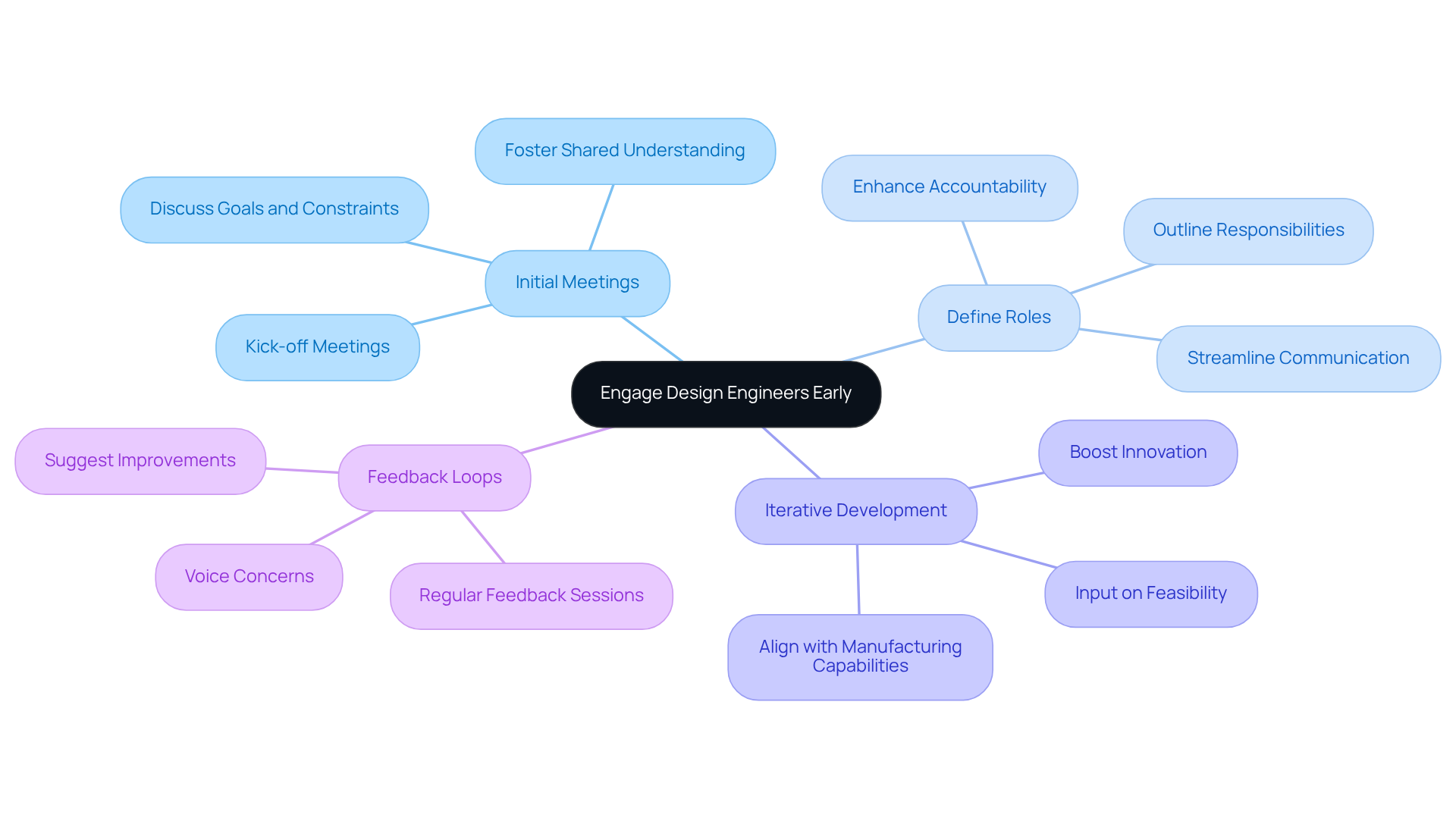
Engaging product design engineer companies at the outset of the product development process can significantly enhance project outcomes. Key strategies for early engagement include:
Involving product design engineer companies early enables organizations to recognize challenges sooner, resulting in more innovative and compliant healthcare products. Statistics indicate that projects with early contractor involvement (ECI) experience fewer changes during construction and achieve better quality solutions, underscoring the value of this collaborative approach.
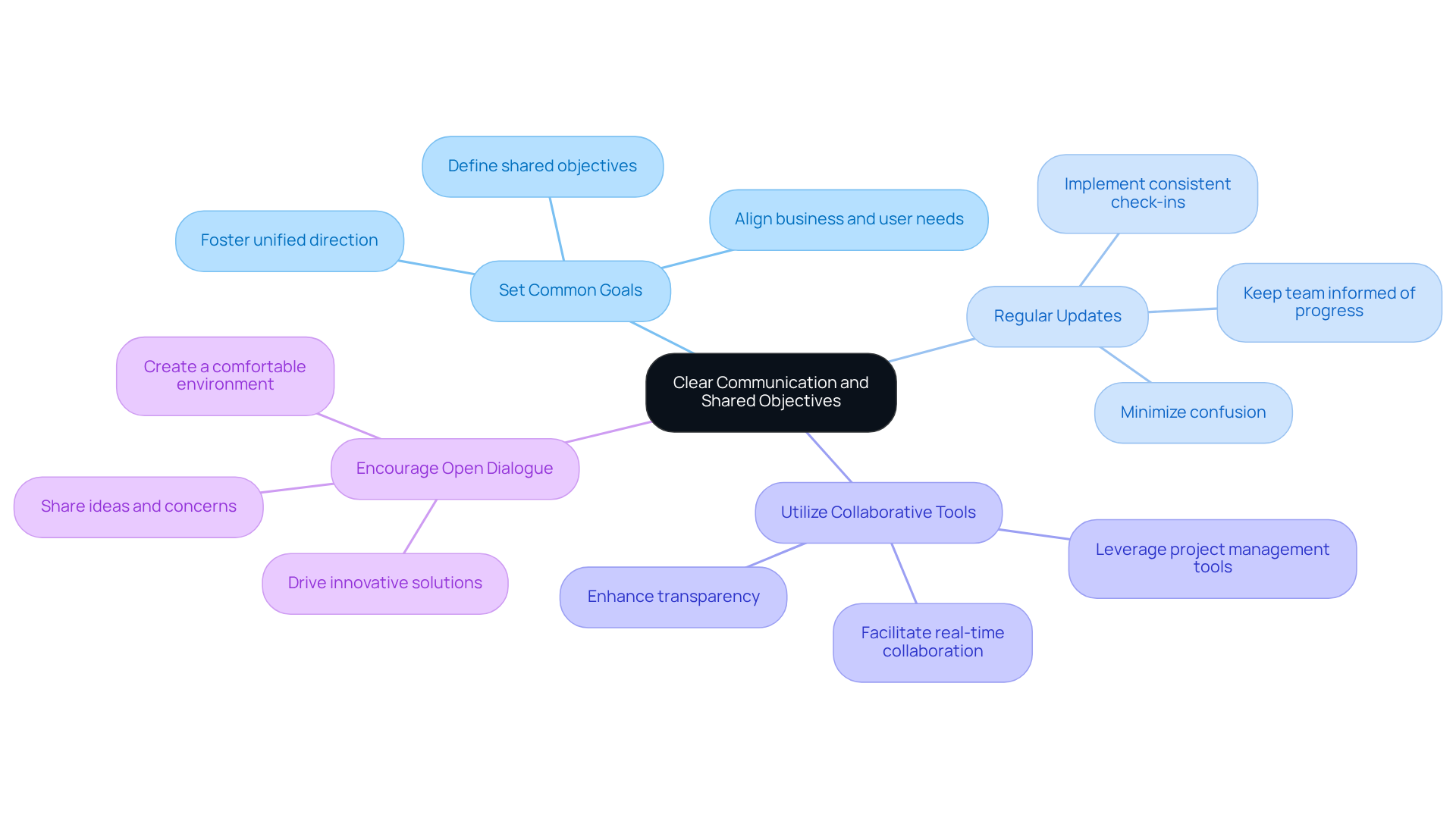
Efficient communication is essential for productive teamwork in healthcare equipment development. To establish clear communication and shared objectives, consider the following best practices:
By prioritizing clear communication and shared objectives, teams can work more cohesively, reducing misunderstandings and enhancing overall project efficiency.
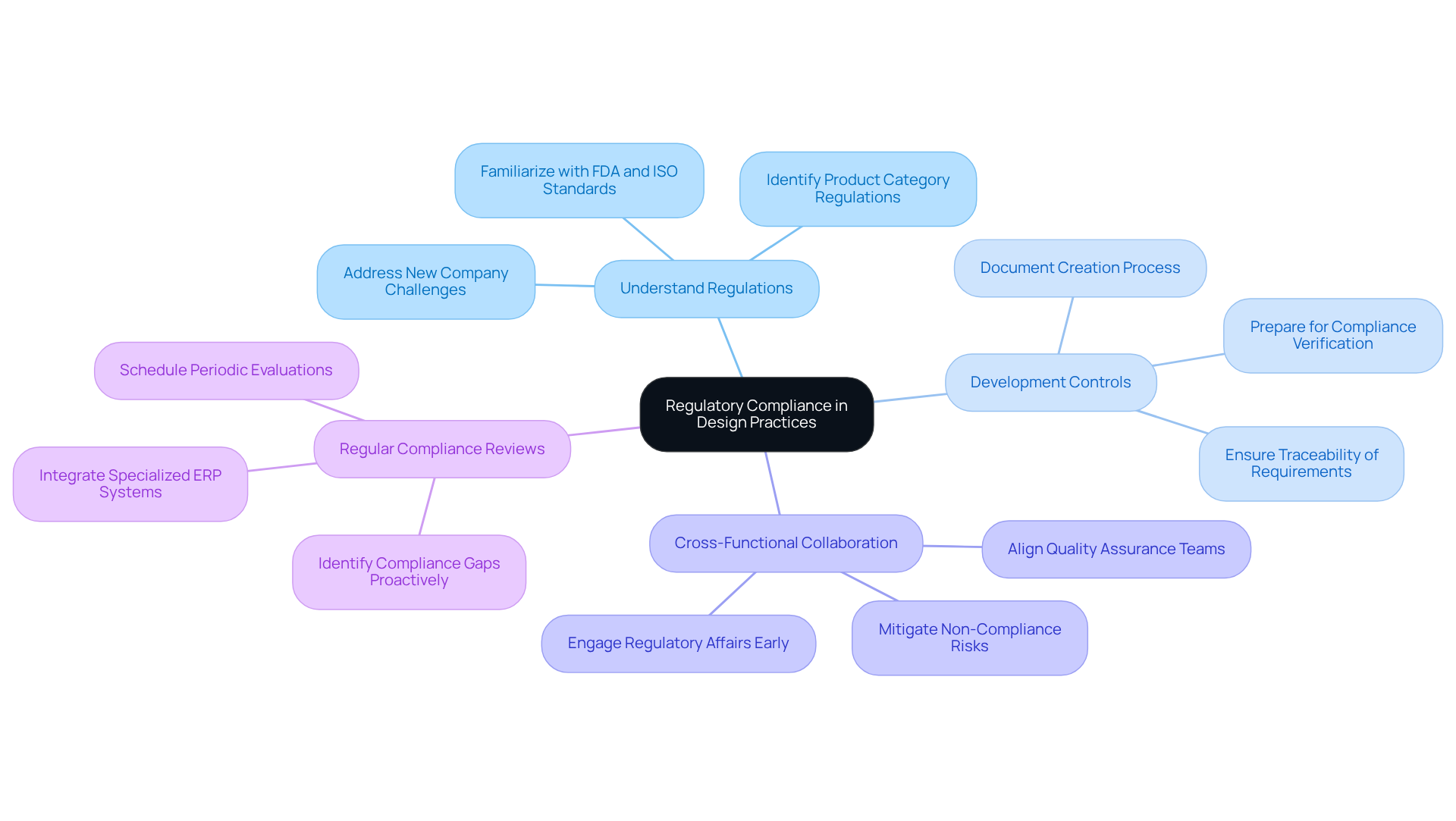
Regulatory compliance is an essential aspect of medical device development that must be integrated into the creation process from the outset. Here are best practices for ensuring compliance:
By embedding compliance into development practices, companies can streamline the approval process and enhance product safety and effectiveness. Furthermore, without appropriate ERP support tailored to industry needs, manufacturers encounter significant operational risks that could jeopardize their compliance efforts.
Iterative feedback and testing mechanisms are crucial for enhancing medical device development and ensuring alignment with user needs. The following best practices can effectively implement these mechanisms:
Prototype Early and Often: Develop prototypes at various stages of the design process to gather feedback and validate design choices. Early prototyping significantly enhances the final product's quality and safety, as changes are easier and less costly to implement in the initial phases.
User Testing: Conduct user testing sessions to gain insights into usability and functionality. The input gathered during these sessions allows developers to make necessary modifications, ultimately resulting in safer and more effective products. Research indicates that incorporating user insights can improve device acceptance and usability.
Feedback Loops: Establish organized feedback loops where team members can provide input on iterative changes. This approach helps clarify misunderstandings and inconsistencies in requirements early on, keeping the team focused on critical issues and enhancing overall product safety.
Document Changes: Maintain comprehensive records of feedback and subsequent modifications to track progress and inform future iterations. This documentation is vital for ensuring compliance with regulatory standards and improving design control.
By adopting an iterative approach, teams can enhance product quality, minimize rework, and accelerate time to market, ultimately leading to successful medical devices that address real-world needs.
Engaging effectively with product design engineering companies is essential for the successful development of medical devices. Understanding the pivotal role that design engineers play - from concept development to regulatory compliance - enables organizations to significantly enhance their product outcomes. This collaboration fosters innovation and ensures that products meet stringent industry standards and user needs.
Key strategies highlighted in this article include:
These practices promote a cohesive team environment, streamline processes, and ultimately lead to higher quality products that are both compliant and market-ready. Furthermore, integrating regulatory considerations from the outset is crucial for navigating the complexities of medical device development.
In a rapidly evolving healthcare landscape, prioritizing collaboration with product design engineers is not merely beneficial; it is vital. Organizations must take proactive steps to incorporate these best practices into their development processes, ensuring they harness the full potential of engineering expertise. By doing so, they can drive innovation, improve patient outcomes, and maintain a competitive edge in the growing medical device market.
What is the role of product design engineers in medical device development?
Product design engineers act as a bridge between innovative concepts and practical applications in medical device development. They translate user needs and regulatory requirements into actionable design specifications, ensuring products meet market demands and compliance standards.
How do product design engineers contribute to concept development?
They effectively translate user needs and regulatory requirements into design specifications, leveraging AI-assisted engineering to enhance the development process and prepare products for future challenges.
What technical expertise do product design engineers possess?
They have a solid understanding of engineering principles, ensuring that concepts are innovative and manufacturable while adhering to stringent industry regulations such as FDA and ISO standards.
How do product design engineers collaborate with other teams?
They work closely with cross-functional teams, including regulatory affairs, quality assurance, and manufacturing, to ensure that every aspect of the product is coordinated, which is essential for meeting project timelines and budgets.
What is the importance of prototyping and testing in medical device development?
Product design engineers create prototypes and conduct evaluations to validate assumptions, ensuring that the final product is safe and effective for end users. This process facilitates a smoother transition from concept to market.
What impact do product design engineers have on the success rates of healthcare instruments?
Initiatives that incorporate their expertise from the outset typically achieve higher compliance and market readiness, with successful projects showing a 90% success rate in delivering products on time and within budget.
What is the projected growth of the global healthcare equipment creation and development services market?
The market was projected to reach approximately USD 12.79 billion by 2025, with an expected compound annual growth rate (CAGR) of 14.42% from 2026 to 2033.
Why is early engagement of design engineers important in the development process?
Engaging design engineers early enhances project outcomes by allowing for a shared understanding of goals, defining roles, promoting iterative development, and establishing feedback loops, which leads to more innovative and compliant healthcare products.
What strategies can be employed for early engagement of design engineers?
Key strategies include scheduling initial meetings to discuss project goals, clearly defining roles and responsibilities, promoting iterative development for input on feasibility, and establishing regular feedback sessions throughout the development cycle.
