Define M2M: A Step-by-Step Guide for Medical Device Manufacturers
Learn how to define M2M technology and its impact on medical device manufacturing.

Effective medical device development relies on a careful balance of innovation, compliance, and collaboration. As the industry navigates an ever-changing regulatory landscape and faces increasing demands for quality, understanding best practices in medical device contract design becomes essential. This article explores key strategies that not only improve product development but also ensure compliance with stringent standards.
However, as organizations pursue excellence, they may encounter challenges in aligning design and manufacturing processes. Identifying these hurdles and implementing effective solutions is crucial for fostering a culture of continuous improvement.
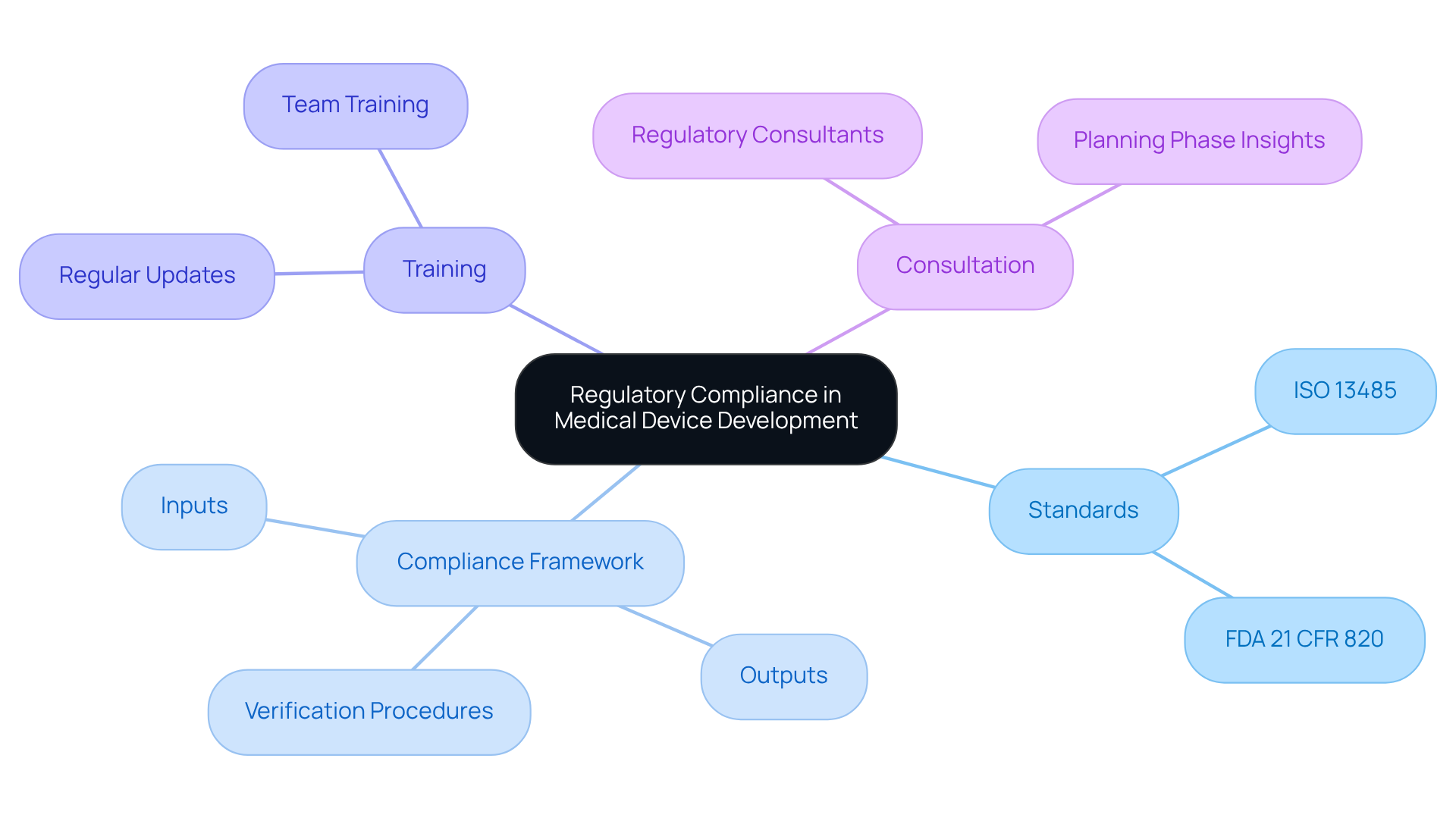
A comprehensive understanding of the regulatory landscape is essential for effective medical device development. Familiarity with standards such as ISO 13485, which governs quality management systems, and the FDA's 21 CFR 820, which regulates product development, is imperative. Organizations should establish a compliance framework at the outset of the development phase, ensuring that all inputs, outputs, and verification procedures align with these regulatory requirements.
Regular training and updates on regulatory changes are crucial for keeping teams informed and compliant. Additionally, collaborating with regulatory consultants during the planning phase can provide valuable insights and facilitate approval submissions, ultimately enhancing the likelihood of successful market entry.
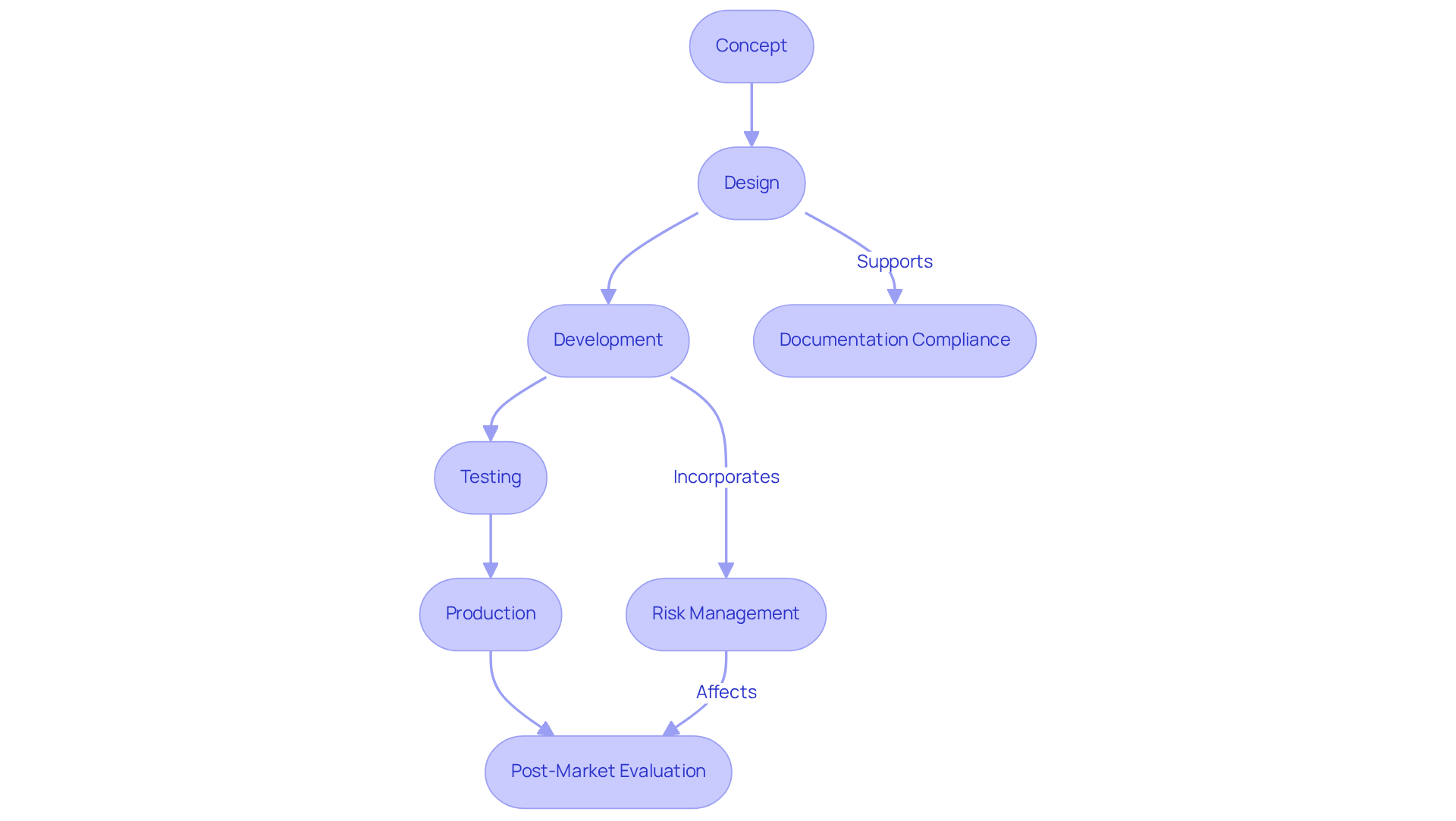
A systematic development process is essential for the successful creation of healthcare devices, which includes the medical device contract design and encompasses clearly defined phases from concept to production. Methodologies such as medical device contract design ensure thorough documentation and review at each phase, which enhances traceability and accountability.
Voler Systems offers comprehensive documentation compliance support, assisting startups in navigating the regulatory challenges inherent in medical device contract design within the medical technology industry. Tools like Gantt charts and project management software effectively visualize timelines and delineate responsibilities among team members.
Regular evaluations of the layout and milestone assessments are crucial for the early detection of potential issues, enabling prompt modifications to the project path. Furthermore, incorporating risk management practices throughout the medical device contract design development process is vital for mitigating unforeseen challenges, ultimately leading to higher success rates in project delivery.
Organizations that adopt these structured methods are better positioned to meet regulatory standards and avoid costly recalls, as evidenced by case studies highlighting the consequences of inadequate control.
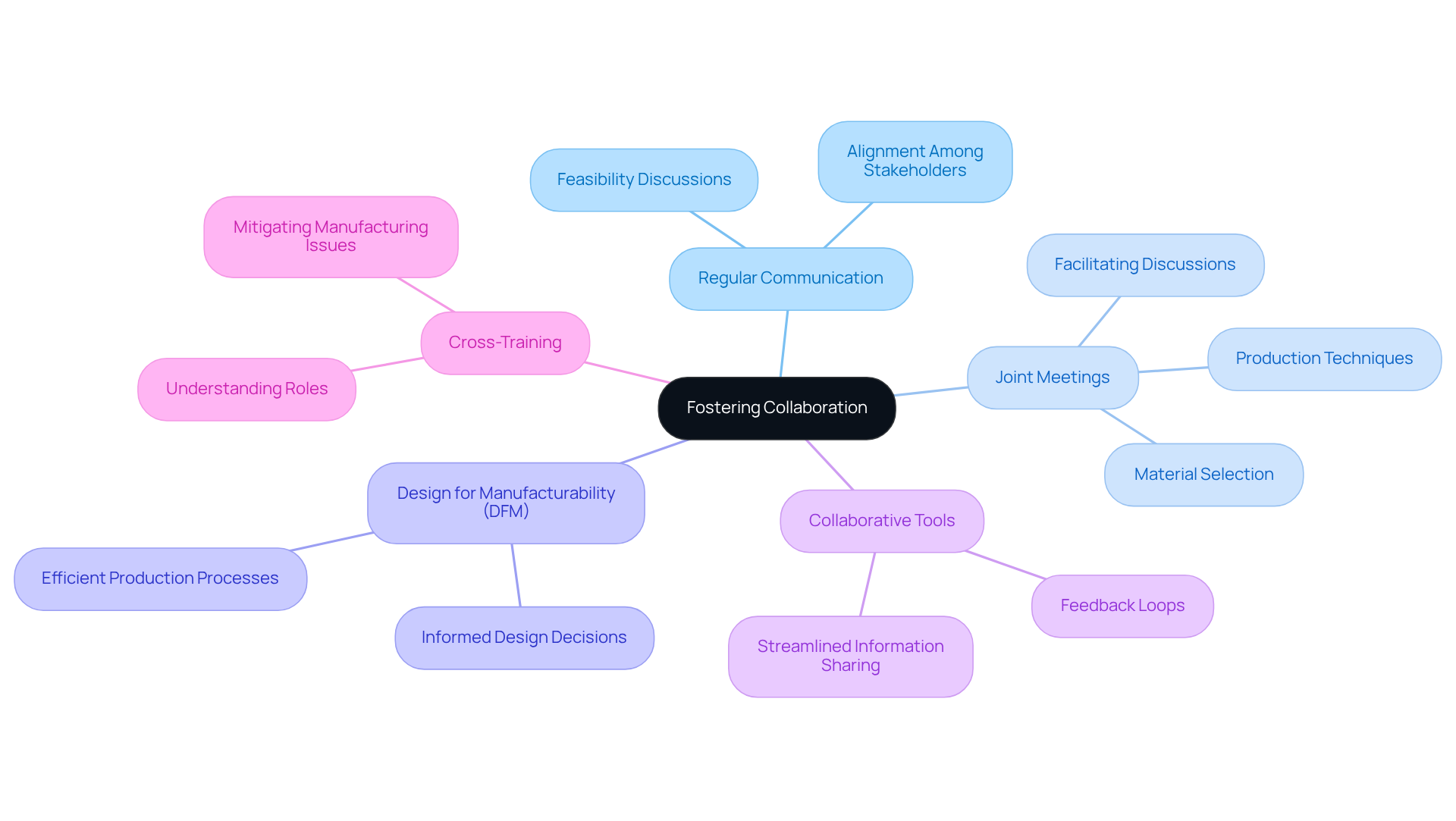
To enhance collaboration, establishing regular communication channels between the creative and manufacturing teams is essential. Joint meetings facilitate discussions on feasibility, material selection, and production techniques, ensuring alignment among all stakeholders. Adopting a Design for Manufacturability (DFM) approach informs design decisions that align with manufacturing capabilities, ultimately resulting in more efficient production processes. The use of collaborative tools, such as shared digital platforms, streamlines information sharing and feedback loops, thereby improving overall project coordination.
Encouraging team members to engage in cross-training cultivates a deeper understanding of each other's roles, which is vital for cohesive product development. This collaborative environment not only mitigates potential manufacturing issues but also promotes the development of innovative healthcare products that adhere to medical device contract design and meet stringent industry standards.
As Dave Fromm, Chief Operations Officer, articulates, "Through early and ongoing cross-functional collaboration among all engineers on the project, today’s medtech designers can create tomorrow’s most impactful healthcare devices, including innovations in medical device contract design that are miniature in size but massive in clinical value."
Furthermore, Voler Systems leverages advanced embedded systems architecture, employing FPGA and AI technologies to enhance battery life in wireless therapeutic equipment. Their expertise in electronic motion detection and ECG solutions further underscores their commitment to developing innovative health equipment that complies with industry standards.
Integrating diverse principles can further enhance the functionality and manufacturability of healthcare products, ensuring they meet the evolving demands of the industry.
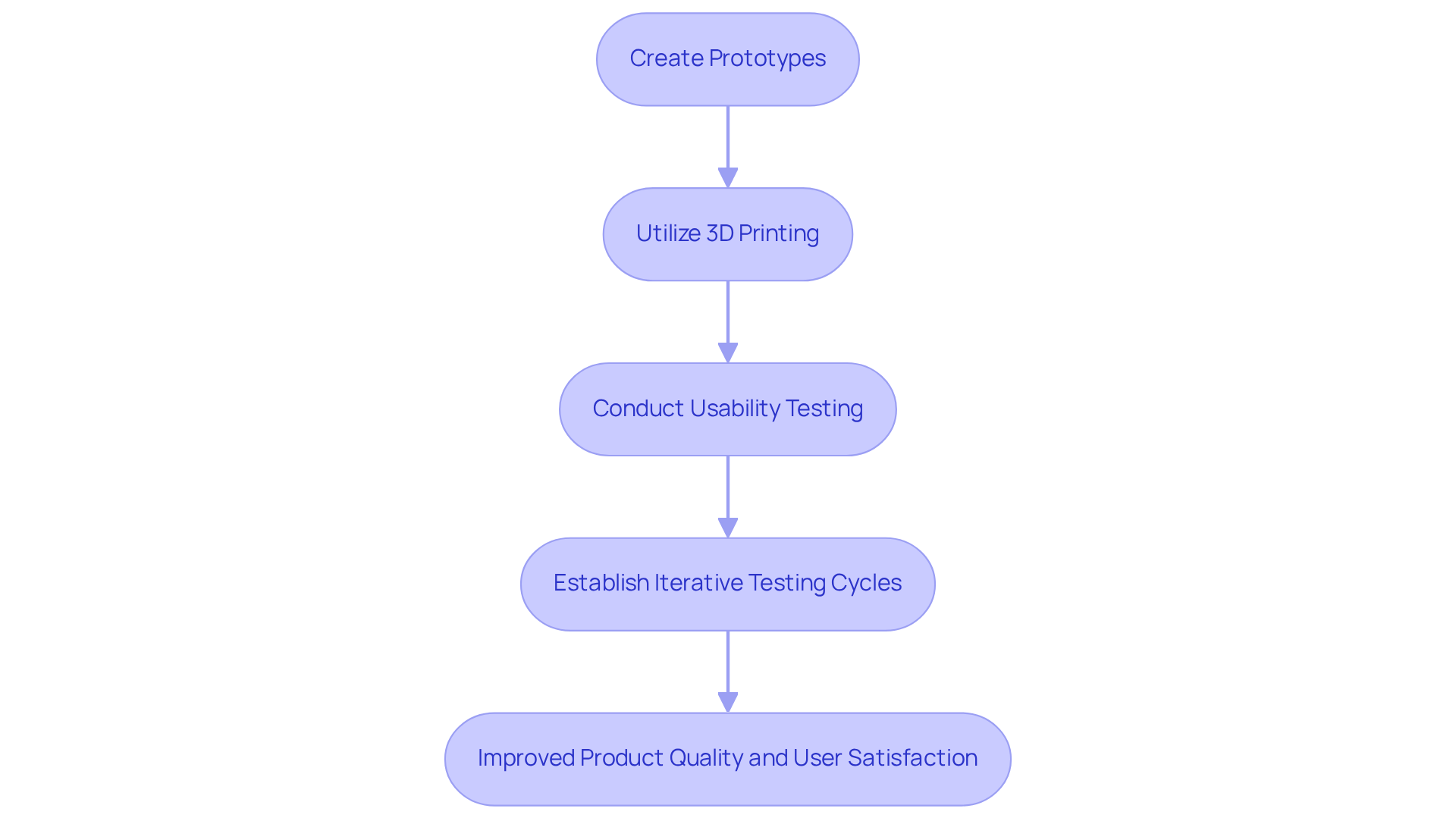
Prototyping plays a crucial role in the development of medical apparatus, facilitating the creation of physical models that can be rigorously assessed for functionality and usability. The incorporation of 3D printing technology significantly expedites this phase, enabling rapid iterations informed by user feedback. For example, hospitals and surgical centers are increasingly utilizing 3D printing to manufacture patient-specific surgical tools and anatomical models, thereby enhancing the validation process and shortening the time to market.
Conducting usability testing with actual users provides invaluable insights into design enhancements, ensuring that products effectively address the needs of both patients and clinicians. Establishing iterative testing cycles, where each prototype is evaluated and findings are meticulously documented, promotes continuous improvement. This approach not only elevates product quality but also ensures that the final product closely aligns with user expectations, ultimately leading to improved patient outcomes and satisfaction.
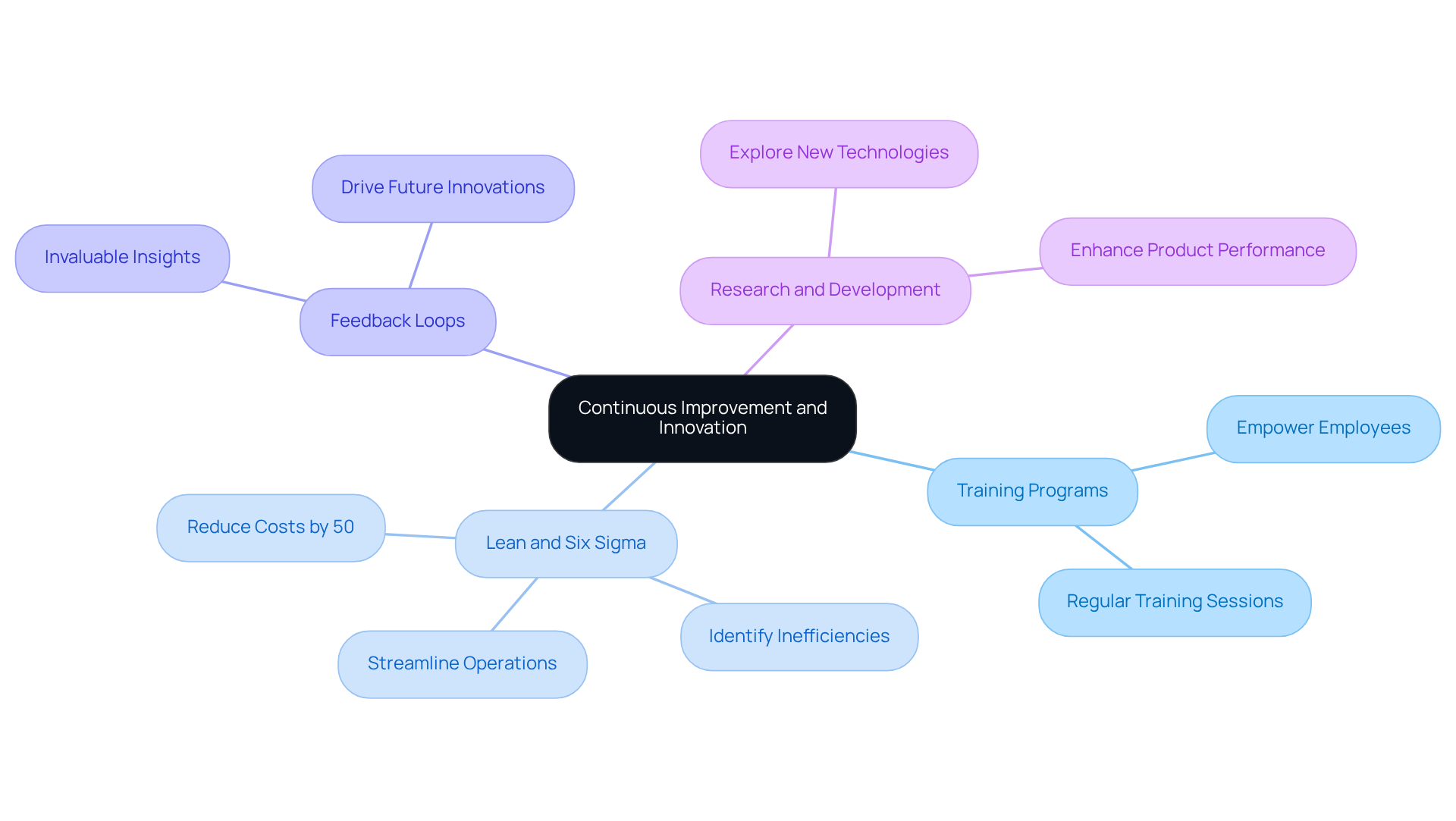
To cultivate a culture of continuous improvement, organizations must implement regular training programs that empower employees to identify and propose enhancements to processes and products. Lean and Six Sigma methodologies are pivotal in pinpointing inefficiencies and streamlining operations. Studies indicate that companies employing these frameworks can achieve up to a 50% reduction in manufacturing costs.
Establishing a robust feedback loop with end-users is essential, as it provides invaluable insights that drive future innovations. Furthermore, investing in research and development is critical for exploring new technologies and materials that can significantly enhance product performance.
By prioritizing innovation and leveraging Lean and Six Sigma principles, organizations can ensure their medical device contract design not only complies with current standards but also proactively addresses future market needs, positioning themselves for success in 2026 and beyond.
In conclusion, effective medical device contract design is fundamentally rooted in a thorough understanding of regulatory compliance, structured design processes, and a culture that promotes collaboration and innovation. Successfully navigating the intricate landscape of medical device development necessitates that organizations prioritize these best practices. This approach not only ensures that products meet stringent standards but also addresses the diverse needs of users.
Key insights emphasize the critical importance of:
Each of these components plays a vital role in mitigating risks, enhancing product quality, and ultimately facilitating successful market entry.
As the medical device industry continues to evolve, it is imperative for organizations to remain proactive in adopting these best practices. By investing in training, embracing innovative technologies, and nurturing a culture of collaboration, companies can not only comply with existing regulations but also anticipate future market demands. This unwavering commitment to excellence will position them favorably for success in the dynamic landscape of medical device development.
Why is understanding regulatory compliance important in medical device development?
A comprehensive understanding of the regulatory landscape is essential for effective medical device development, as it ensures that all aspects of the development process align with necessary standards and regulations, such as ISO 13485 and the FDA's 21 CFR 820.
What are ISO 13485 and FDA's 21 CFR 820?
ISO 13485 governs quality management systems for medical devices, while FDA's 21 CFR 820 regulates the product development process for medical devices in the United States.
How can organizations ensure compliance during medical device development?
Organizations should establish a compliance framework at the outset of the development phase, ensuring all inputs, outputs, and verification procedures align with regulatory requirements. Regular training and updates on regulatory changes are also crucial.
What role do regulatory consultants play in medical device development?
Collaborating with regulatory consultants during the planning phase can provide valuable insights and facilitate approval submissions, enhancing the likelihood of successful market entry.
What is the significance of structured design processes in healthcare device development?
A systematic development process is essential for successful healthcare device creation, ensuring thorough documentation, review, traceability, and accountability throughout all phases from concept to production.
How does Voler Systems assist startups in medical device contract design?
Voler Systems offers comprehensive documentation compliance support to help startups navigate the regulatory challenges of medical device contract design within the medical technology industry.
What tools can help manage timelines and responsibilities in medical device development?
Tools like Gantt charts and project management software are effective for visualizing timelines and delineating responsibilities among team members.
Why are regular evaluations and milestone assessments important?
Regular evaluations and milestone assessments are crucial for early detection of potential issues, allowing for prompt modifications to the project path and enhancing the likelihood of successful project delivery.
How can risk management practices benefit the medical device development process?
Incorporating risk management practices throughout the development process is vital for mitigating unforeseen challenges, ultimately leading to higher success rates in project delivery.
What are the consequences of inadequate control in medical device development?
Organizations that do not adopt structured methods may face costly recalls, as evidenced by case studies highlighting the negative outcomes of inadequate control.
