5 Best Practices for Collaborating with Product Design Engineer Companies
Discover best practices for collaborating with product design engineer companies in...

Understanding the complex landscape of medical device development reveals a critical truth: effective product planning is essential for success. This structured approach encompasses vital elements such as:
All aimed at ensuring that innovative medical solutions meet both regulatory standards and market demands. With the rapid evolution of technology and regulatory frameworks, manufacturers must navigate these complexities to achieve timely and successful market entry.
Planning for medical devices represents a structured approach that defines, develops, and manages the lifecycle of a medical item from inception to market launch. This process encompasses essential stages that are considered elements of product planning, including:
Effective planning, encompassing the elements of product planning, is crucial as it ensures that the final product not only meets market demands but also adheres to stringent regulatory standards, facilitating successful commercialization. This approach transcends mere creation; it aligns with strategic business objectives and guarantees the offering's viability in a competitive landscape.
Voler Systems exemplifies the significance of thorough product planning in healthcare technology. By leveraging expert electronic design services and AI-assisted engineering, they assist manufacturers in accelerating the development of wearable devices, heart pumps, and liquid biopsy platforms. Successful case studies indicate that companies which meticulously evaluate user needs and compliance requirements often experience smoother market entry and enhanced customer satisfaction. The integration of AI-driven insights into planning further enhances efficiency, enabling manufacturers to adapt swiftly to evolving market conditions and compliance changes. Ultimately, the elements of product planning for offerings, bolstered by Voler Systems' comprehensive IoT design consulting, serve as a cornerstone of innovation in the healthcare sector, directly impacting the success and sustainability of new solutions in the marketplace.
The planning for goods in the healthcare equipment sector began in the early 20th century with the establishment of initial oversight frameworks. In the absence of stringent regulations, this development often raised significant safety concerns. As technology advanced and the demand for medical equipment grew, oversight organizations such as the FDA introduced more rigorous guidelines to ensure safety and effectiveness.
The adoption of quality management systems (QMS) and standards like ISO 13485 has markedly enhanced the elements of product planning, highlighting the importance of thorough documentation, risk management, and user-centered design.
Today, the elements of product planning represent a complex discipline that integrates technical, compliance, and market considerations, reflecting the intricate demands of modern healthcare. This evolution underscores the critical need for manufacturers to navigate regulatory environments effectively, particularly as the FDA continues to adapt its frameworks to accommodate advancements in digital health and AI-driven technologies.
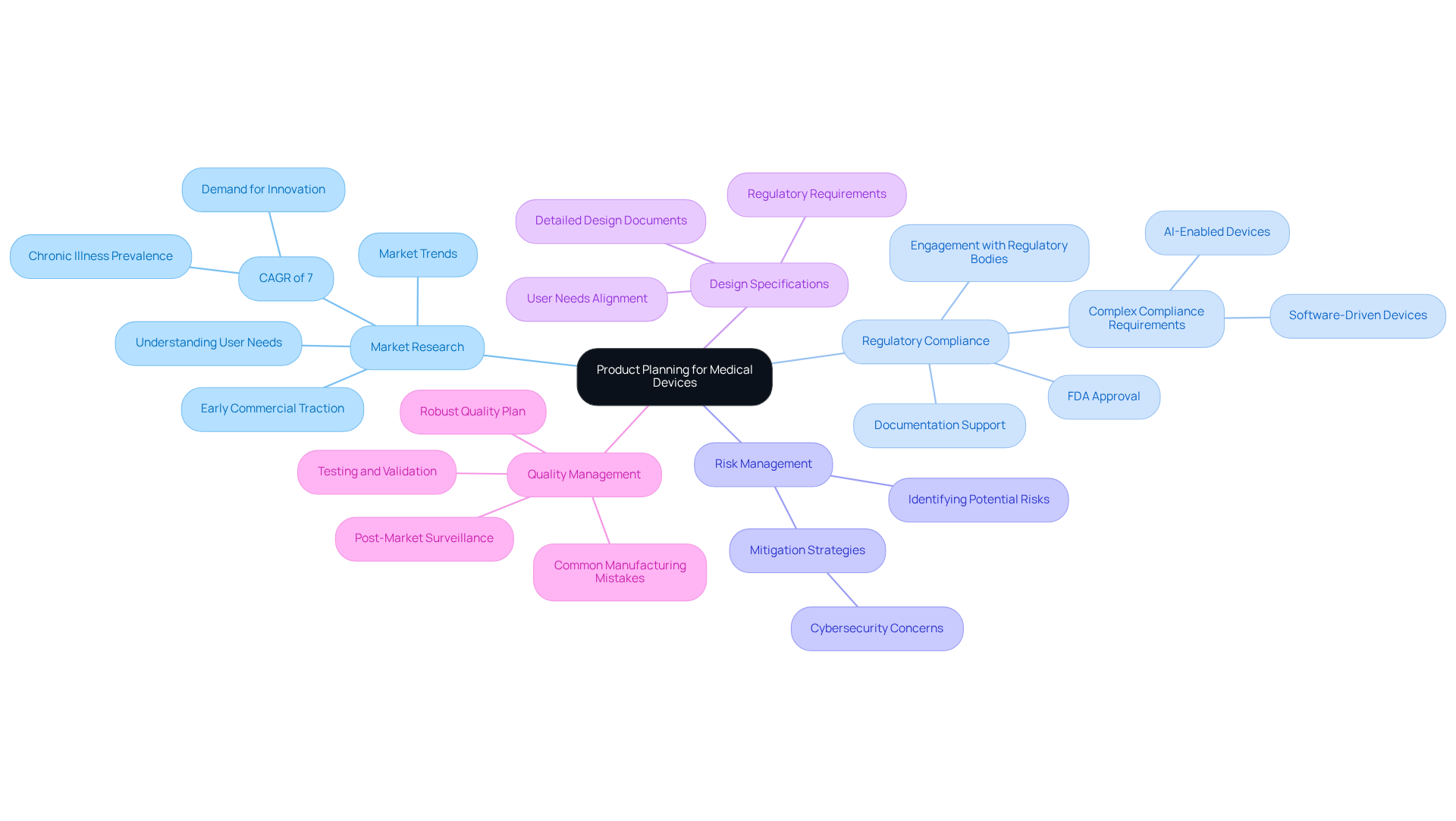
Key components of product planning for medical devices encompass several essential elements:
Market Research: Understanding user needs and market trends is vital for informing product design. The medical equipment market is anticipated to expand at a compound annual growth rate (CAGR) of 7%, driven by the increasing prevalence of chronic illnesses and the demand for innovative solutions. Companies that prioritize early commercial traction and demonstrate clinician interest are more likely to attract investment and succeed in this competitive landscape.
Regulatory Compliance: Adhering to necessary regulations and standards, such as FDA approval, is crucial. The evolving legal environment requires manufacturers to navigate complex compliance requirements, particularly for software-driven and AI-enabled devices. Successful strategies include early engagement with regulatory bodies and thorough documentation to streamline the approval process. Voler Systems offers expertise in documentation compliance support, assisting startups in meeting these requirements efficiently.
Risk Management: Identifying potential risks associated with the product and implementing effective mitigation strategies is essential. This includes addressing cybersecurity concerns, which have become increasingly important as connected devices proliferate in healthcare settings.
Design Specifications: Creating detailed design documents that outline the product's features and functionalities ensures clarity and alignment among stakeholders. This step is critical for maintaining focus on user needs and regulatory requirements throughout the development process.
Quality Management: Establishing a robust quality plan that includes testing, validation, and post-market surveillance is crucial for ensuring safety and effectiveness. Continuous monitoring and adherence to quality standards help maintain compliance and enhance user trust. Identifying common mistakes in establishing manufacturing tests is vital, as these can significantly affect the quality and efficiency of electronic product design.
The elements of product planning play a crucial role in steering the development process, ensuring that the final offering is not only safe and effective but also ready for the market, addressing the increasing demand for innovative healthcare solutions.
Real-world examples of effective planning for items are prominently illustrated in the development of wearable medical devices, particularly continuous glucose monitors (CGMs). Businesses like Dexcom have skillfully navigated the complexities of planning by conducting thorough market analysis to identify user requirements, ensuring compliance with standards, and employing robust risk management strategies.
For instance, Dexcom's CGM underwent extensive user testing, which was crucial in refining its design and functionality. This meticulous approach not only ensured adherence to legal standards but also significantly enhanced the patient experience. Notably, Dexcom's recent launch of the G7 CGM, cleared for 15 days of continuous monitoring, exemplifies successful product planning that integrates user feedback and regulatory considerations.
Furthermore, innovative wearable health instruments, such as a calf-worn device designed for motion and circumference monitoring in knee replacement rehabilitation, underscore the importance of compliance with healthcare equipment standards. Voler Systems' expertise in healthcare product design further emphasizes the necessity of managing compliance from prototype to production.
These examples illustrate the critical nature of the elements of product planning, which must seamlessly integrate user feedback, regulatory considerations, and technical specifications to ultimately lead to successful outcomes in the medical device sector.
Effective product planning serves as the backbone of successful medical device development, ensuring that products not only meet market needs but also comply with stringent regulatory standards. This structured approach encompasses critical elements such as:
By integrating these components, manufacturers can navigate the complexities of the healthcare landscape, ultimately leading to innovative solutions that enhance patient care.
Key arguments throughout the article highlight the importance of thorough planning, illustrated by real-world examples such as Dexcom's continuous glucose monitors. These case studies demonstrate how meticulous attention to user feedback and compliance can significantly impact product success and market entry. The evolution of product planning, from early oversight frameworks to modern practices, underscores the necessity for manufacturers to adapt to changing regulations and technological advancements.
In conclusion, the significance of robust product planning in the medical device industry is paramount. As the demand for innovative healthcare solutions continues to rise, manufacturers must prioritize effective planning strategies to ensure their offerings are safe, effective, and market-ready. Embracing the elements of product planning not only fosters innovation but also enhances patient outcomes, ultimately shaping the future of healthcare technology.
What is product planning in the context of medical devices?
Product planning for medical devices is a structured approach that defines, develops, and manages the lifecycle of a medical item from its inception to market launch.
What are the essential stages included in product planning?
The essential stages of product planning include market research, user needs evaluation, compliance with regulations, and design specifications.
Why is effective product planning important?
Effective product planning is crucial as it ensures that the final product meets market demands and adheres to regulatory standards, facilitating successful commercialization and aligning with strategic business objectives.
How does Voler Systems contribute to product planning in healthcare technology?
Voler Systems assists manufacturers by leveraging expert electronic design services and AI-assisted engineering to accelerate the development of medical devices such as wearable devices, heart pumps, and liquid biopsy platforms.
What benefits do companies experience from meticulous product planning?
Companies that meticulously evaluate user needs and compliance requirements often experience smoother market entry and enhanced customer satisfaction.
How does AI integration enhance product planning?
The integration of AI-driven insights into product planning enhances efficiency, enabling manufacturers to adapt swiftly to evolving market conditions and compliance changes.
What role does product planning play in the innovation of healthcare solutions?
The elements of product planning, supported by comprehensive IoT design consulting, serve as a cornerstone of innovation in the healthcare sector, directly impacting the success and sustainability of new solutions in the marketplace.
