Healthcare and technology go hand in hand to deliver better healthcare to patients worldwide. The current COVID-19 global pandemic and advancements in AI are top drivers encouraging healthcare organizations to adopt IoT (internet of things) medical devices.
Last year, around 87% of healthcare organizations have adopted IoT technology. By 2025, experts predicted that the wearable technology market will reach $74.03 billion, a significant increase from its valuation in 2019 of $27.91 billion.
Very simply, a medical device is any instrument, apparatus, implement, appliance, machine, implant, in vitro reagent or calibrator, material, software, or another similar or related article for diverse uses, including monitoring, supporting, control, and provision of health. Internet of things simply means the use of electronic devices that acquire or monitor data and are connected to either public or private networks, allowing them to initiate certain actions.
From Novelty to Standard Practice
Since March there has been a radical change in remote monitoring influenced by the Covid-19 pandemic. In merely 3 months, remote monitoring went from fringe technology to mainstream due to the need to monitor patients without a physical visit to a doctor or hospital. FDA implemented temporary regulations aiding the use of these devices, and two new payment codes were put in place for remote monitoring.
On August 3, President Donald Trump, who got diagnosed with Covid-19 early in October, signed a new Executive Order that further expands access to telehealth services. The order in the White House fact sheet states “the availability of certain telehealth services after the current public health emergency ends.”
Companies making these devices are overwhelmed with the demand. These companies need assistance from expert electronic design teams specializing in the Internet of Things (IoT) and Wearable Device Meetup to develop their products on a budget, efficiently and push it to the market faster.
FDA Regulation Changes
FDA issued a guidance for Industry and Food and Drug Administration Staff titled Enforcement Policy for Non-Invasive Remote Monitoring Devices Used to Support Patient Monitoring During the Coronavirus Disease 2019 (COVID-19) Public Health Emergency (Revised) on March 20 to address the Coronavirus Disease 2019 (COVID-19) public health emergency.
This policy is intended to remain in effect only for the duration of the public health emergency related to COVID-19 declared by the Secretary of Health and Human Services (HHS) on January 31, 2020, effective January 27, 2020, including any renewals made by the HHS Secretary per section 319(a)(2) of the Public Health Service (PHS) Act (42 U.S.C. 247d(a)(2)).
The policy aims to provide a policy to help expand the availability and capability of non-invasive remote monitoring devices to facilitate patient monitoring while reducing patient and healthcare provider contact and exposure to COVID-19 for the duration of the COVID-19 public health emergency.
This new policy’s scope applies to the legally marketed non-invasive remote monitoring devices listed in Table 1 that measure or detect common physiological parameters and that are used to support patient monitoring during the COVID-19 public health emergency:
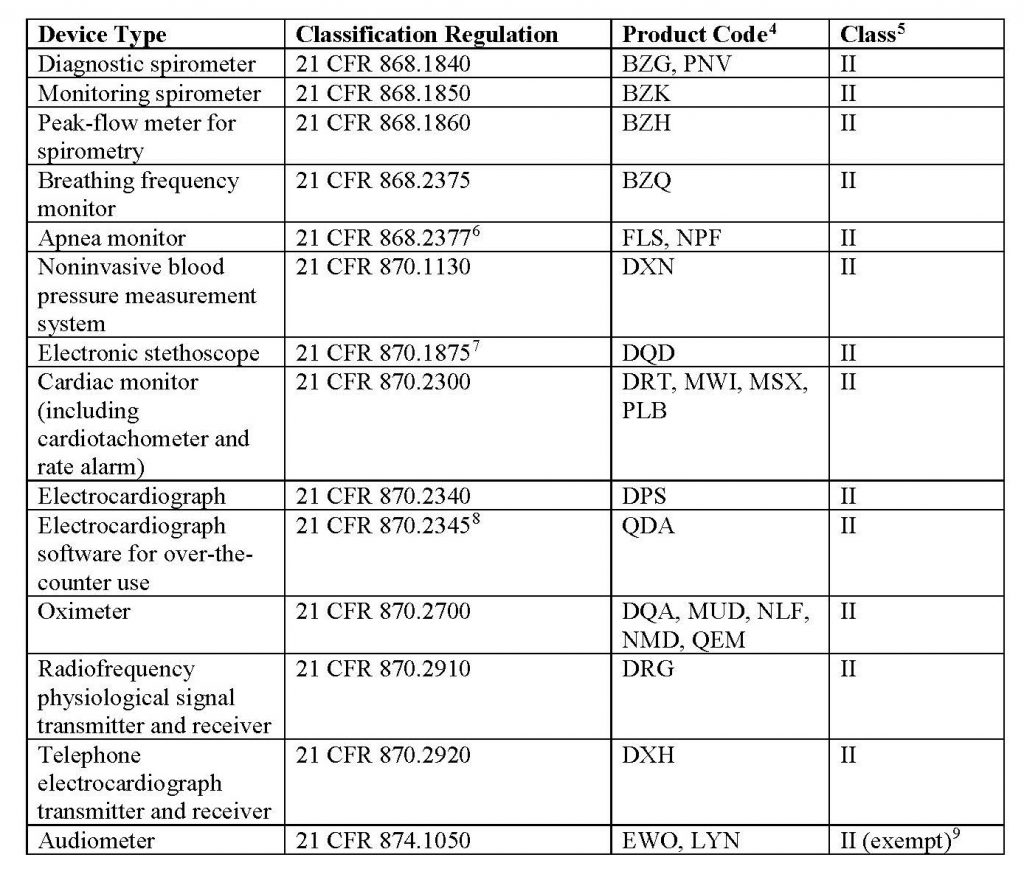
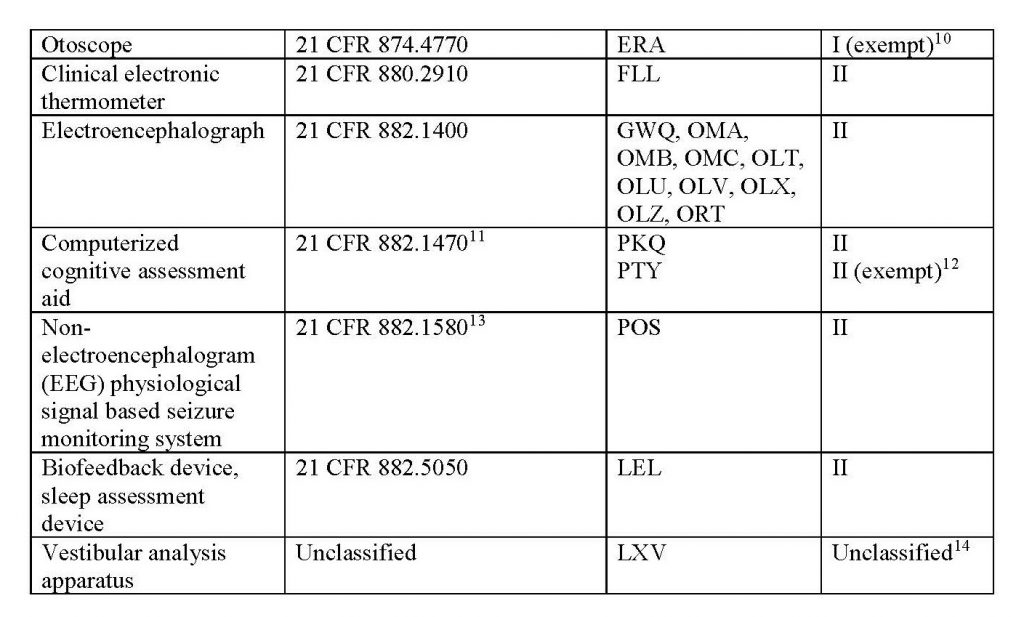
Learn more about FDA’s updated regulations through its official website.
Integrating IoT into Medical Devices
Before the era of IoT, doctor-patient interactions were restricted to physical visits, tele, and text communications, limiting the doctor’s ability to monitor a patient’s health and suggest time-sensitive treatments. The introduction of IoT in the healthcare sector caused patient care to undergo an incredible shift, making care accessible to all. Faster internet, technological advancements, better analytics, an escalating number of competitors in the space furthered IoT healthcare projects to make a positive impact on the healthcare industry.
Adoption of these devices by healthcare providers was slow, however. Getting lots of data from devices of uncertain quality can increase a doctor’s workload and add liability if something is missed. Also, it’s hard to collect data from different devices into one place, because they have proprietary interfaces. Some doctors did adopt this technology and found it was valuable. With the advent of the COVID-19 crisis healthcare providers needed a way to monitor patients with chronic disease without in-person visits. Suddenly the value of remote patient monitoring was recognized, and it has become mainstream. Another value is only just becoming important: it saves money.
Some regions, such as the US, leads in healthcare IoT and in leveraging health-related data derived from IoT devices. We recently saw a record number of vendors entering the IoT market space with IoT medical devices in the 2020 Consumer Electronic Event (CES). Devices include care robots, medwands, monitoring devices, insoles, intelligent home camera systems for tracking seniors at home, and, of course, smartwatches and speakers.
What Devices are in the Medical IoT?
Most IoT in healthcare is centered around improving patient care through remote monitoring and telemonitoring, which applies as the main application in the broader scope of telemedicine. A secondary area with many initiatives includes tracking, monitoring, and maintenance of assets, using IoT sensors. This is utilized on the level of medical devices and healthcare assets, the patient level, and the non-medical asset level (e.g. hospital/clinic building assets).
There is a vast landscape of healthcare stakeholders and possibilities with IoT healthcare projects, including personal healthcare, healthcare insurance, the pharmaceutical industry, RTHS, healthcare building facilities, smart beds, robotics, biosensors, smart pills, anything remote, and the various medical specializations, activities and even disease treatment – the possibilities are endless both for the healthcare industry and device designers/manufacturers.
Voler Systems’ IoT Healthcare Projects
Voler Systems recently created a Somnology prototype project for Somnology Inc. Voler Systems was able to fix and deliver the prototype where two prior firms failed.
Don Aoki, Product Development head at Somnology Inc. stated “I have high confidence in Voler’s engineering acumen, customer service, and integrity. In short, they are an outstanding team that I endorse wholeheartedly.”
Voler Systems is also currently developing a heart monitor that will collect data from patients at home. The product is similar to a Holter monitor, but boasts smaller form and convenience, allowing it to collect data for a much longer time.
End Note
Medical device providers looking to design and release their product to the market should avoid the mistakes medical device startups make, including cramming features, lack of research, not considering FDA requirements, and others. Voler Systems offers full-service R&D consulting from concept and design to the production of medical devices for human use. Since 1979, clients have turned to Voler Systems for device design and development for reliable new products and test systems involving sensors and measurement electronics.
Voler Systems has demonstrated these medical device design and development capabilities by delivering quality products, including wearables, motion control, software, and wireless design, proof of concept, test equipment – all complying with federal and European requirements. Voler’s design experience includes popular devices with partners such as Intel, Qualcomm, Microchip, Intel, LinkLabs, and others.