How IoT Changing Healthcare Today | Voler Systems
Healthcare and technology go hand in hand to deliver better healthcare to patients...
According to the Centers for Disease Control and Prevention (CDC), an estimated 37 million people in the United States have chronic kidney disease (CKD). Of that 37 million, as many as 9 out of 10 people don't know they have it.
Unfortunately, that results in the disease's continued progression and ongoing kidney function decline.
For roughly 2% of patients with CKD, the disease progresses to end-stage renal disease (ESRD). The kidneys no longer function at this point, requiring chronic dialysis and life-saving organ transplants.
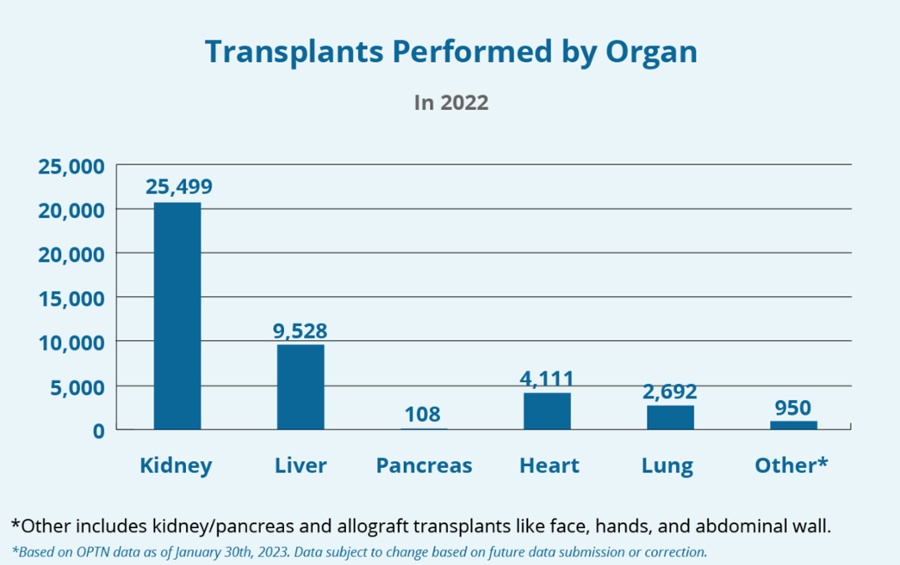
As of 2020, more than 800,000 people in the U.S. have ESRD, with approximately 71% on dialysis. Generally, a kidney transplant is the preferred treatment for ESRD. Kidneys are the most commonly transplanted organ; however, the number of patients on the transplant waiting list dramatically outnumbers the number of available donor organs.
Photos Credit: HRSA Organ Donor.gov
As a result, ESRD patients have no choice but to get dialysis as they wait for a kidney donor. While dialysis can prolong a patient's life, current standards of care present many risks that can negatively impact the quality of life and overall life expectancy.
Visionary Medical Therapies (VMT) aims to change that with revolutionary new technology that can lower the risk of dialysis complications.
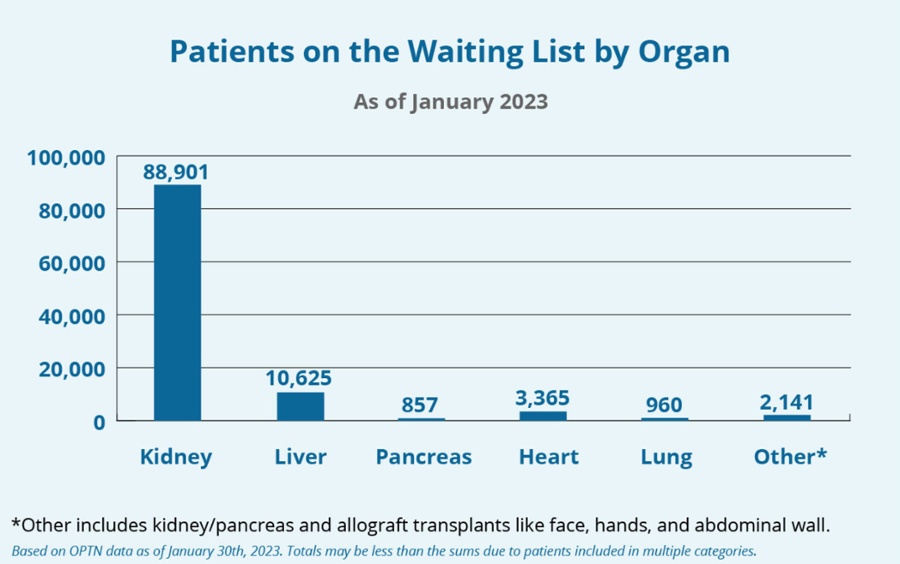
Organ donation is on an upward trend, but it's still not enough to meet the needs of ESRD patients. According to data provided by the Organ Procurement and Transplant Network (OPTN), the U.S. transplant system administered by the United Network for Organ Sharing (UNOS), there are more than 104,000 organ transplant candidates in the United States as of February 2023.
Almost 89,000 of those candidates need a kidney.
The average timeframe for a kidney can be between three and five years at most transplant centers, but that could increase in the future.
A new person gets on the transplant list roughly every nine minutes, and medical researchers believe that the prevalence of ESRD will increase substantially through 2030, creating a more significant burden on the transplant system.
Patients with ESRD must rely on dialysis to stay alive while waiting for an available transplant. Many forms of dialysis exist, but approximately 55% of ESRD patients have implanted arteriovenous grafts (AVGs).
Grafts don't require maturation like arteriovenous fistulas, and they may be the only option for those with limited life expectancy or medical comorbidities.
But AVGs aren't without their issues. They have a six-month failure rate of 50% and a primary patency rate of 58% at six months. AVGs have an artificially shortened lifespan, and a significant contributor to that is improper re-access.
A finite number of implantation sites exist in the body, and each can only be used once. The net result is that ESRD patients die prematurely on the transplant list as they exhaust their AVG options while waiting for a donor organ.
Visionary Medical Therapies is developing new technologies to improve vascular access for ESRD dialysis patients. The seasoned management team includes individuals with decades of experience advancing medical technology. With a proven track record of success from ideation to exit, VMT is well-equipped to tackle the many long-overlooked issues of ESRD dialysis treatment.
VMT's Helios™ AVG is an early-stage device that's patent-protected. It's a differentiated medical device that will provide access to the $2.5 billion and growing global vascular access market. It has the potential to improve safety and ease of use while reducing complications linked to current AVG standards of care.
Arteriovenous grafts are synthetic blood vessels connecting arteries and veins. It is a form of hemodialysis access that many patients and providers choose in place of arteriovenous fistulas. The grafts are usually crafted from synthetic materials that are nonimmunogenic and nonthrombotic.
The most dominant material is expanded polytetrafluoroethylene, commonly known as Teflon, due to its anti-thrombotic and self-healing properties.
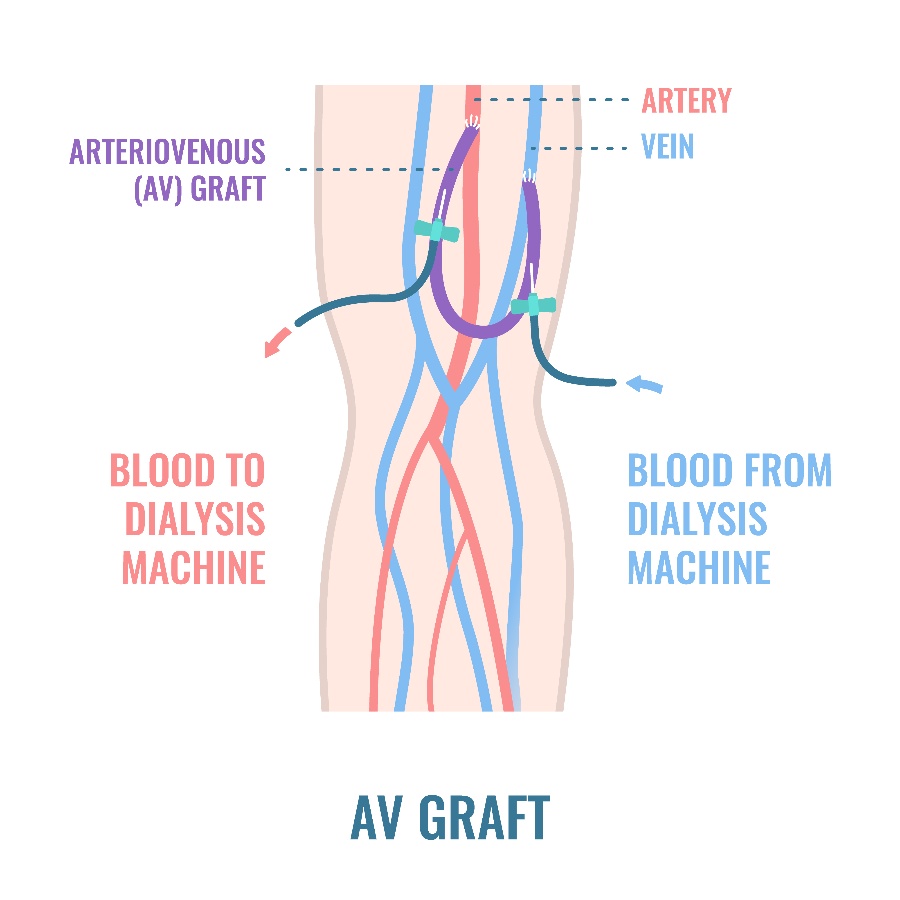
During dialysis treatment, technicians insert needles into the AVG inflow and outflow, connecting them to a dialysis machine for filtering.
The problem with current AVG standards of care is that technicians must operate unguided. These grafts are difficult to see, resulting in inaccurate re-access. It's a painful experience for ESRD patients due to repeated pokes and prods.
Considering that ESRD usually requires three hemodialysis treatments a week, it takes its toll. But that's not all.
Re-access issues also shorten the lifespan of the graft. Most reach their half-life by six months. Failed AVG re-access is associated with systemic complications like collapse, pseudoaneurysms, increased risks of stenosis, and a higher chance of infection. Infections most occur within six months of AVG implantation, putting already-vulnerable patients at risk.
Those complications also come with substantial monetary losses. The average cost of the most widely used grafts is around $1,000. Billions of dollars are spent annually due to malfunctioning vascular access.
For perspective, ESRD patients make up about 1% of individuals on Medicare, but the treatment involved accounts for roughly 7% of the annual Medicare budget.
Patients cannot afford constant AVG complications and repeat treatments due to improper insertion. Not only is it expensive, but there are only a limited number of available implantation spots.
If an ESRD patient remains on the transplant list for many years, there's a real concern about running out of implantation sites.
The VMT Helios solves many issues with today's standard AVG protocols. It's a unique AVG design that facilitates guided access, eliminating the complications associated with failed re-access attempts.
Instead of going in blind, technicians can use the Helios to gain access correctly the first time, reducing risks of failure and creating a more comfortable treatment experience for patients.
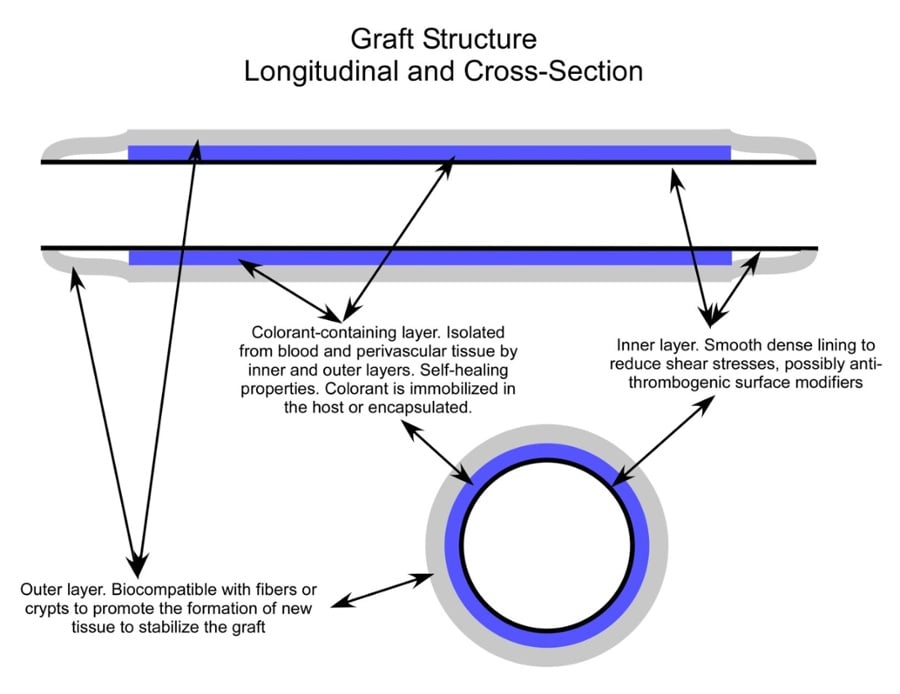
Like other AVG designs, the Helios features material with self-healing properties. The outside is biocompatible with fibers to promote the development of new stabilizing tissue. Meanwhile, the inner layer is dense and smooth to reduce shear stress. An anti-thrombotic coating can retain the benefit of lowered blood clot risks.
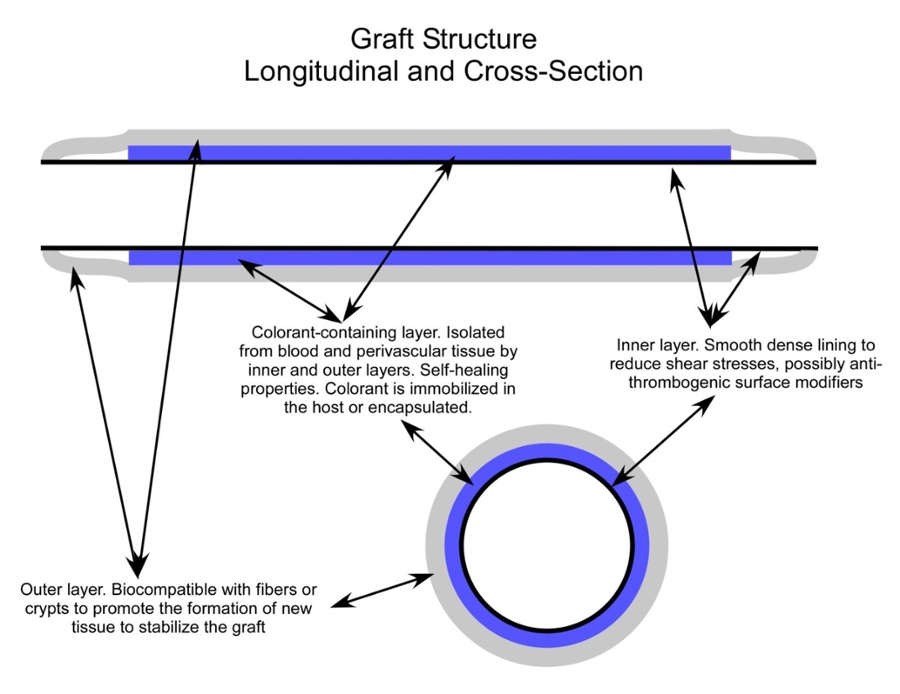
But what makes the Helios different is the encapsulated or immobilized layer of infrared fluorophore colorant. Exposure to short-wave infrared (SWIR) LED lighting allows the fluorophore layer to re-emit light.
This process unveils the location of the Helios AVG, providing technicians with the visual guide they need to cannulate the graft properly.
The images below show examples of how contemporary lasers, optics, and tissue optics interact to make the Helios concept a reality.
 |
 |
 |
On the upper left, we have an image of the back of a hand being illuminated by a commercial VeinViewer™ (Christie Medical). The veins are identified by imaging in the near-infra-red spectral range (> 700 nm), and the positions of the veins are back-projected on the hand in the visible (green) to guide the medical professionals as they place access catheters, etc.
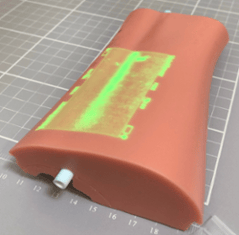
The middle image shows a prototype Helios graft inserted in a silicone radial artery access simulator (Simulab) and imaged by a modified vein viewer. This demonstrates that the Helios graft can be readily identified by equipment that is very familiar in form and function to that already in use in dialysis clinics, phlebotomy departments, emergency departments, ICU, PICU, NICU, etc.
The image on the right shows a second prototype Helios graft being imaged by proprietary fluorescence image-guided surgery (FIGS) equipment developed for the Helios project. Equipment of this type is already in use in surgical departments around the world used to verify perfusion, for example, in surgical flaps in reconstructive surgery and for identification of body lumens in laparoscopic surgery.
The target containing the fluorophore is illuminated in the near-infrared spectral range, as with the vein viewer. But in the case of FIGS, the target is identified by the longer wavelength fluorescence emitted by the fluorophore. It is straightforward to produce an uncluttered background-free image as the excitation and emission wavelengths are quite distinct and are easily separated by optical filters.
Clinical Implementation
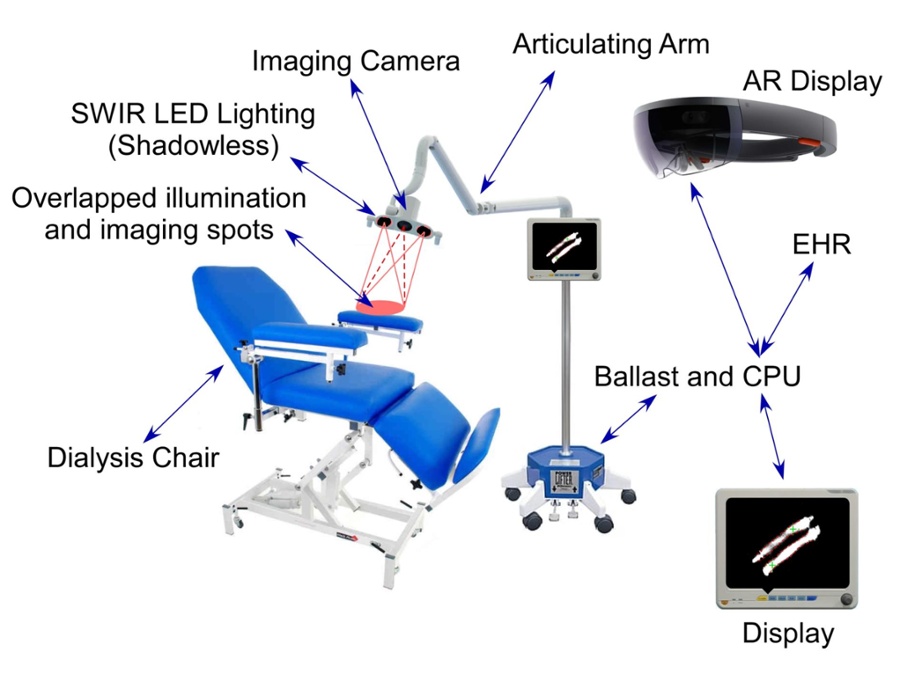
The Helios imaging system has been designed for both conventional vein and Helios graft locations and access guidance to maximize reimbursement opportunities and minimize downtime (the perennial bugaboo of hospital administrators).
The similarity with equipment and techniques already in use will allow medical personnel to cannulate Helios grafts without additional expensive advanced medical imaging.
The technology unveils the location of blood vessels and existing AVGs. However, the Helios AVG, paired with an advanced imaging system, can pave the way for even more effective treatment.
The VMT imaging system can identify the graft, establish borderlines to direct technicians, and even calculate an optimal insertion point. Estimated points can appear through augmented reality (AR) glasses or with back projection, making re-access as straightforward as possible.
Over time, a history of re-access points is generated (the "Stick Map"). The imaging system uses this information to optimize future access points to minimize localized damage, prolong the life of the graft, and decrease damage to the patient.
The average AVG lasts approximately one year before failure due to the many complications. Patients can suffer tremendously during that time, and the risks of further health issues increase. Visionary Medical Therapies hopes to change that. With the Helios AVG and advanced imaging systems, ESRD patients can get effective dialysis with fewer risks and more comfort.
This VMT technology has the potential to help healthcare providers and patients in other areas. For example, it has possible use cases for devices such as chemo ports, PICC lines, swan catheters, etc.
Voler Systems has helped companies like VMT bring innovative medical devices to market for over four decades. Our electronic device design consultants can make your product a reality, from concept to design to production to market.
Contact Voler Systems to discuss your next medical device project.
Disclaimer
Design work on the VMT Helios™ AVG has recently finished, and the device is not yet approved or on the market.
About VMT
VMT is an innovative Med Tech startup dedicated to improving the lives of patients worldwide, starting with the Helios™ AV Access system. For more information or opportunities for investment, please contact Christopher Smith, President VMT, at csmith@visionarymedicaltherapies.com.
Co-authored by John Black: John Black has over 25 years of experience in the medical, scientific / research, and industrial segments of the lasers and photonics industry. He founded Glannaventa to develop an outpatient endoscopic imaging technique to screen for the most lethal histotype of serous ovarian cancer, and through Triermain LLC, also consults with companies in laser design and development, optical coherence tomography (OCT), medical device design and development, image-guided surgery, and optical remote sensing. For more information about John and his work, please visit his website.
