Best Practices for IoT Design Services in Medical Devices
Explore best practices for IoT design services in medical devices to enhance patient care.

Selecting the appropriate embedded development tools is a critical decision that can greatly impact the success of projects, especially in the healthcare sector where accuracy and compliance are essential. This article explores four best practices that assist developers in navigating the intricate selection process, ensuring that tools are aligned with project requirements and constraints. Given the vast array of options available, how can teams effectively traverse this landscape to identify tools that not only fulfill immediate needs but also foster long-term innovation and integration?
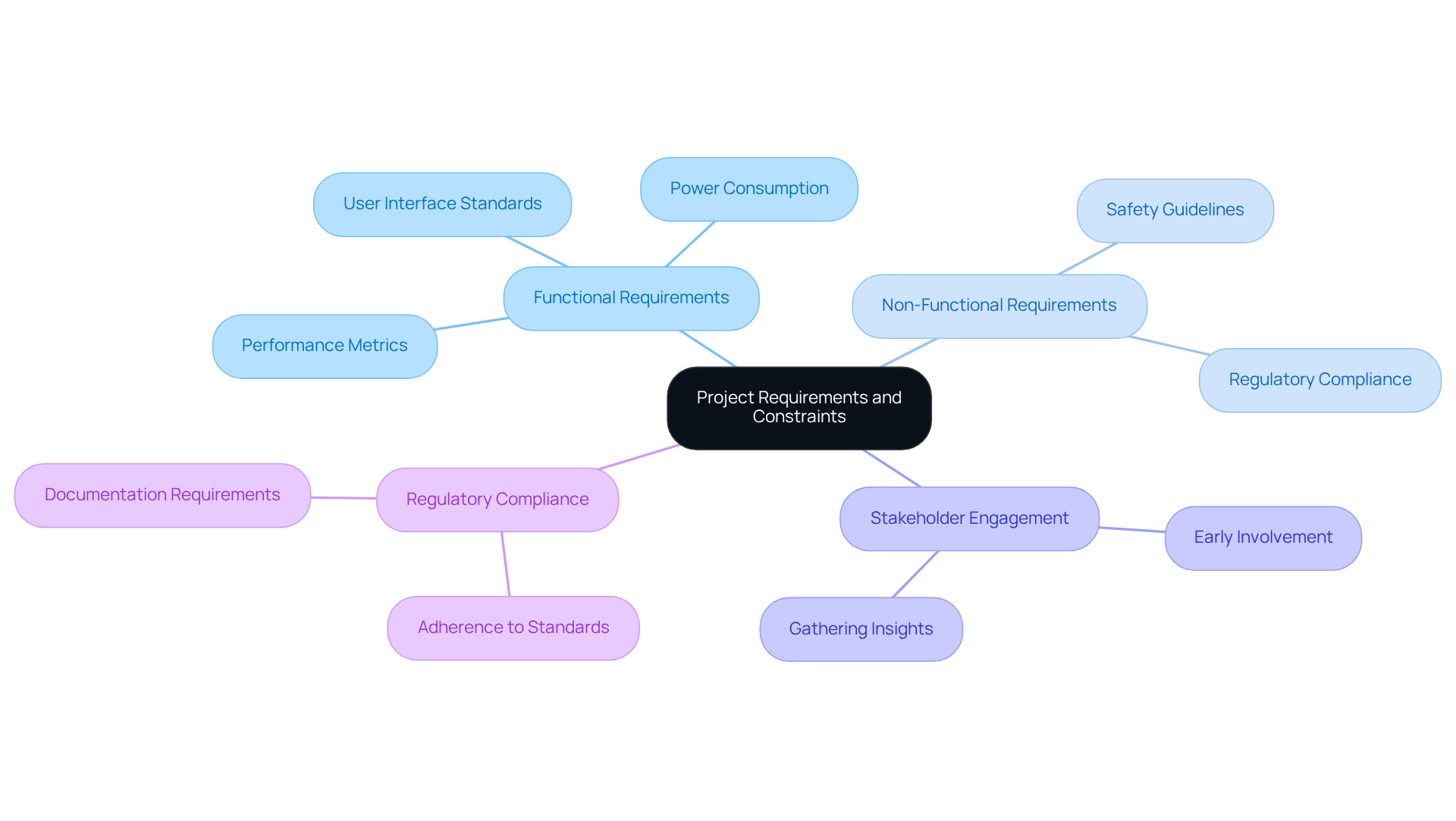
Clearly outlining project requirements and limitations is essential when selecting embedded development tools for healthcare instruments. This process necessitates a comprehensive understanding of both functional and non-functional requirements, which include performance metrics, power consumption, and adherence to regulatory standards. For example, the creation of healthcare instruments often mandates compliance with specific safety guidelines and user interface standards.
Voler Systems has developed hundreds of healthcare tools, such as wearable solutions, heart pumps, and liquid biopsy platforms, leveraging AI-assisted engineering to accelerate product development while improving battery life through innovative power management strategies. Documenting these requirements meticulously allows teams to select resources that align with project needs, thereby mitigating risks and preventing complications during the production phase.
Engaging stakeholders early in this process enriches the requirements gathering phase and provides critical insights that can significantly influence the project's trajectory. As René Zölfl, Global Industry Advisor in Life Science, emphasizes, a thorough understanding of these requirements is vital for ensuring the success and safety of healthcare instruments. Furthermore, recognizing project constraints, such as regulatory compliance and performance limitations, is crucial for the effective use of embedded development tools in system design.
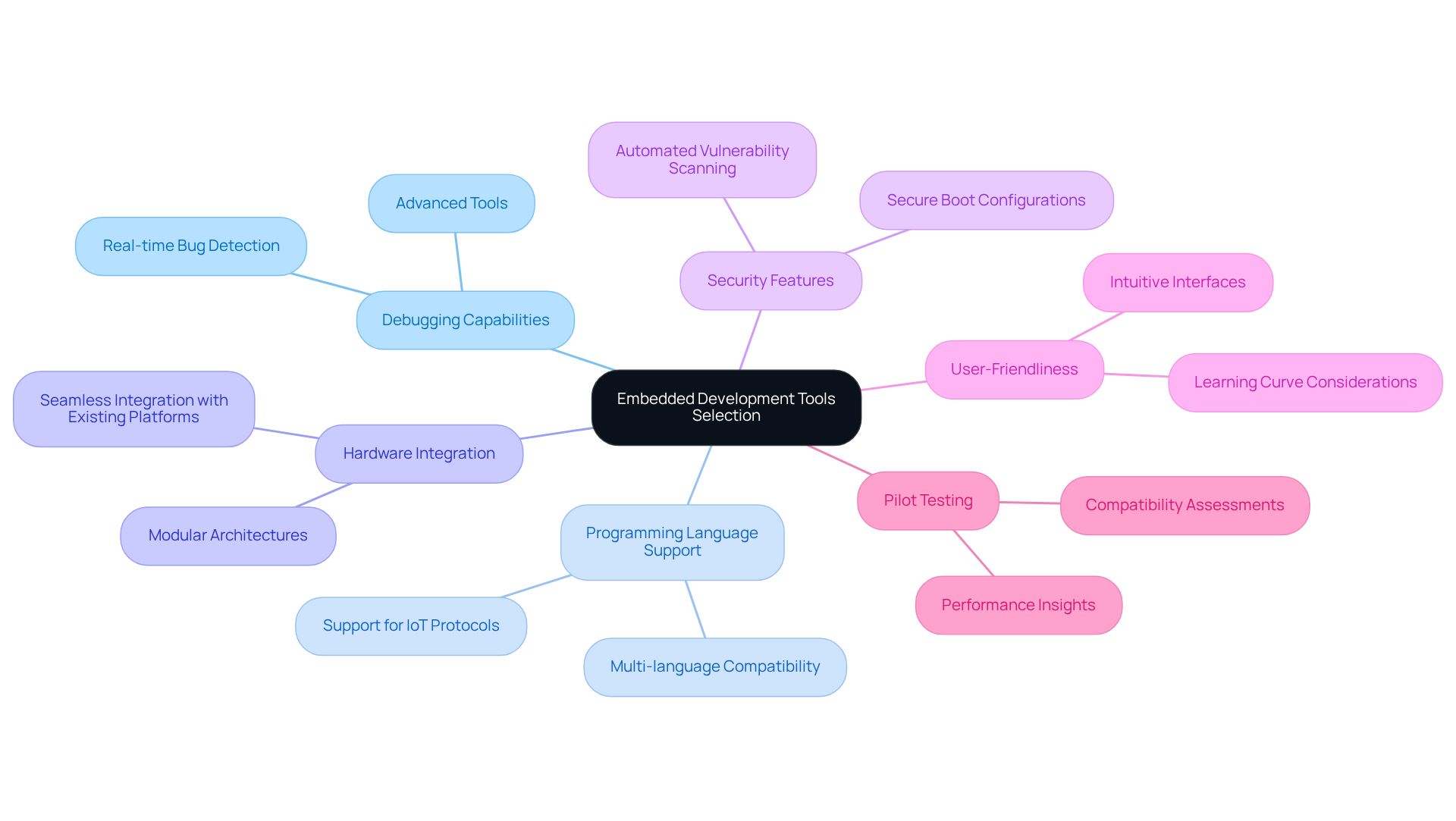
Selecting the right embedded development tools is essential for the success of medical equipment projects. It is important to prioritize embedded development tools that demonstrate robust debugging capabilities, support multiple programming languages, and integrate seamlessly with existing hardware platforms. For instance, when developing IoT devices, it is critical to choose resources that effectively manage wireless communication protocols and data handling.
Data indicates that 68% of IoT attacks stem from insecure firmware, highlighting the necessity for solutions that incorporate automated vulnerability scanning and secure boot configurations to enhance security. Additionally, consider the user-friendliness and learning curve associated with each embedded development tools resource, as these factors significantly influence team productivity.
Conducting pilot tests with selected tools can yield valuable insights into their performance and compatibility with specific project requirements, thereby facilitating a smoother production process.
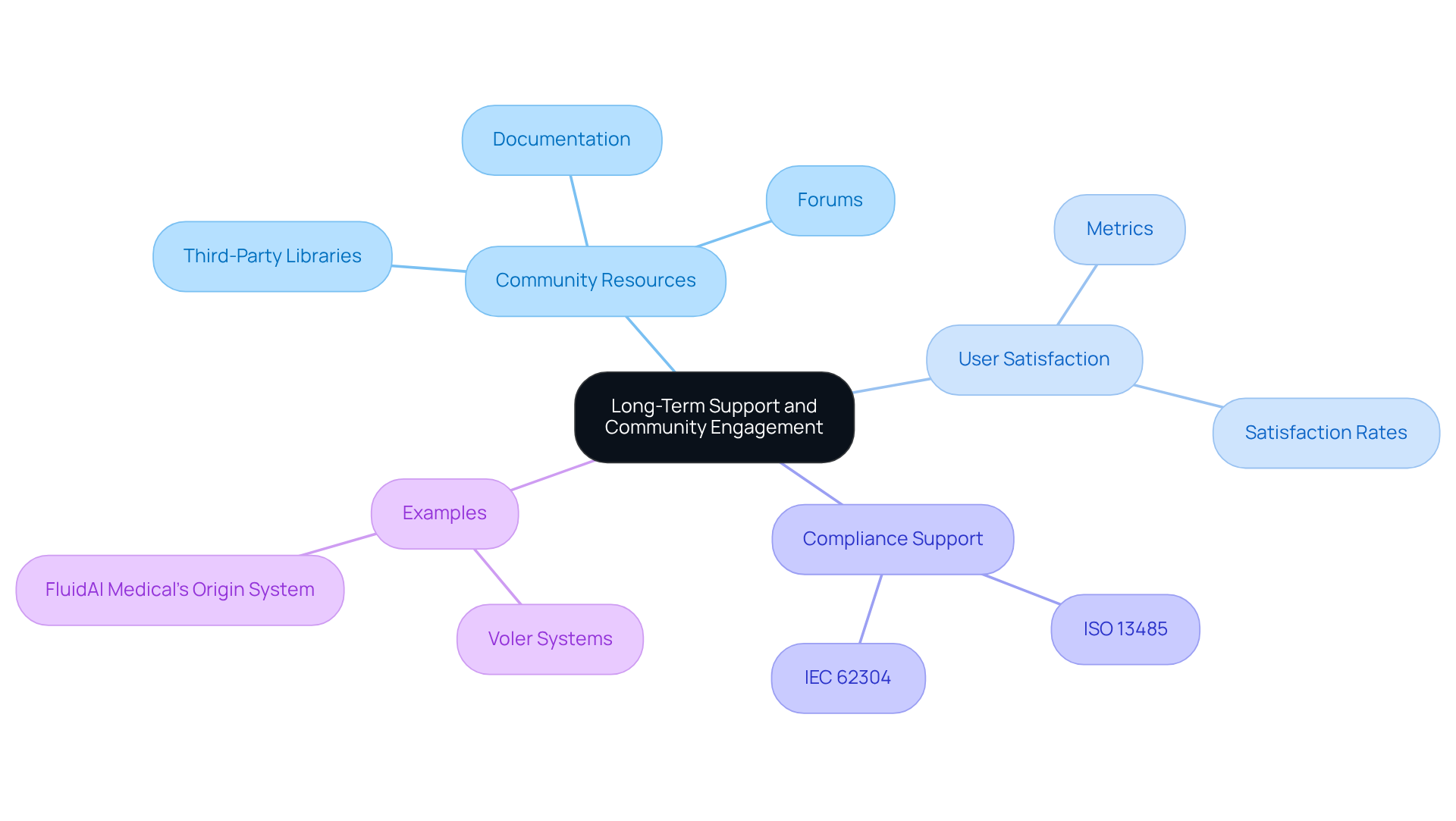
When selecting embedded software solutions, it is essential to consider both long-term support and community involvement associated with each option. Tools supported by active communities typically offer superior resources, including forums, comprehensive documentation, and third-party libraries, which can be invaluable during development. Community engagement metrics reveal that resources with robust user bases often exhibit higher satisfaction rates and better compliance support with industry standards.
Furthermore, it is crucial to ensure that the tools chosen have a transparent roadmap for updates and support, particularly concerning security patches and adherence to regulatory requirements. Tools widely used in the healthcare equipment sector, such as those conforming to ISO 13485 and IEC 62304, may offer enhanced long-term sustainability due to their established user base and ongoing enhancement efforts.
Voler Systems exemplifies this commitment by assisting medical device companies with legacy test equipment, ensuring compliance with necessary standards. This community engagement not only fosters innovation but also ensures that developers have access to the latest advancements and best practices in embedded development tools, as illustrated by Voler Systems' thorough compliance review process for emissions and ESD standards.
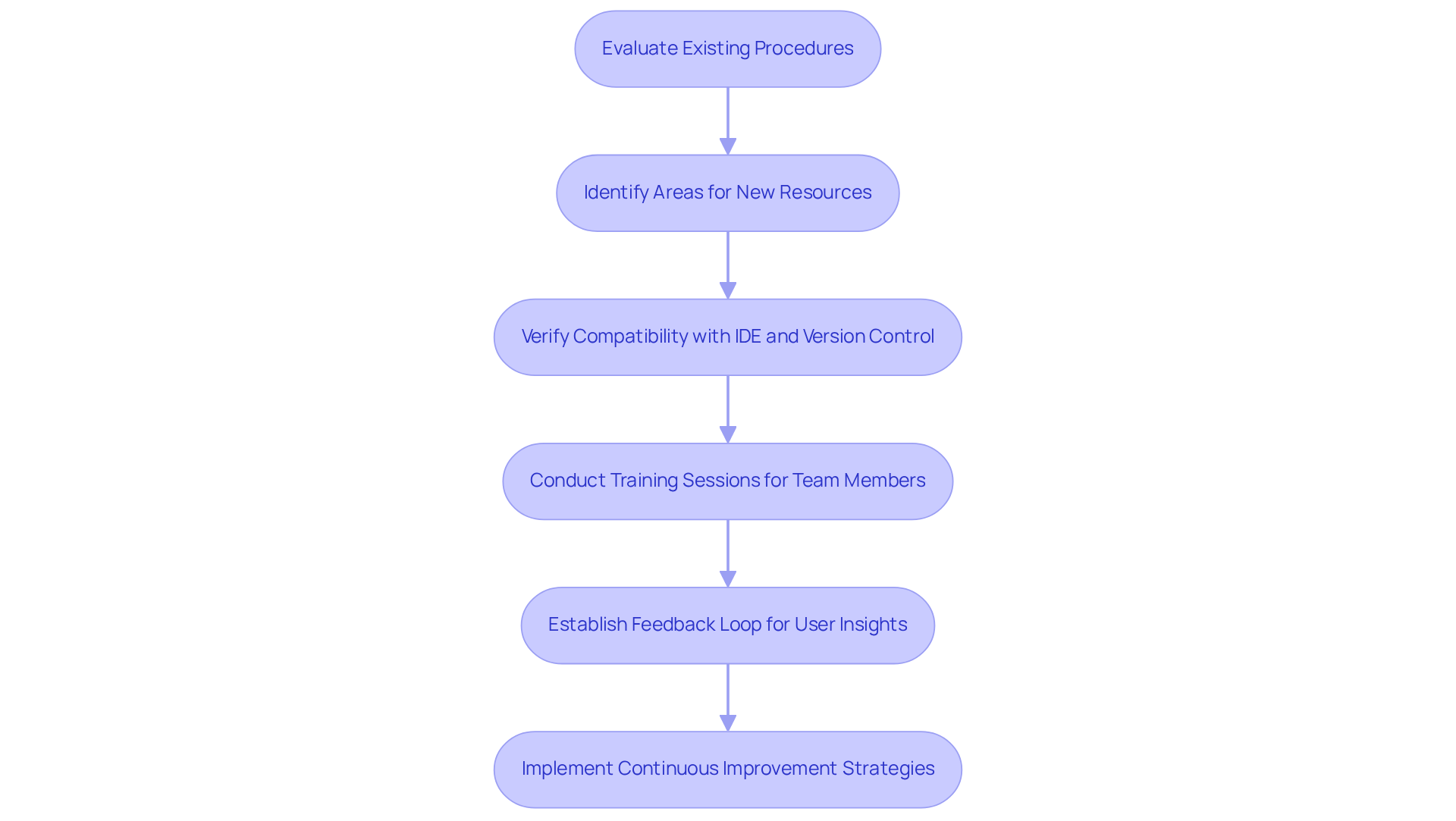
Incorporating embedded development resources into current workflows necessitates meticulous planning and execution. Begin by evaluating your existing procedures to pinpoint areas where new resources can yield significant benefits. For example, when implementing a new Integrated Development Environment (IDE), it is crucial to verify compatibility with your version control system and build processes to prevent disruptions.
Training sessions for team members are vital; they facilitate a smoother transition and empower staff to adapt to new resources effectively. Establishing a feedback loop is essential for collecting user insights throughout the integration process, which enables continuous improvement and necessary adjustments.
Successful case studies in medical device development, such as those from Voler Systems, underscore the challenges of maintaining integrations and illustrate how organizations have effectively incorporated embedded tools, resulting in enhanced productivity and streamlined workflows. By prioritizing these strategies and incorporating critical steps such as thorough planning, stakeholder engagement, and iterative testing, companies can ensure a successful transition to new technologies, ultimately fostering innovation and efficiency in their development processes.
Selecting the appropriate embedded development tools is a critical decision that can significantly impact the success of healthcare projects. A well-defined approach that encompasses understanding project requirements, evaluating tool features, considering long-term support, and integrating these tools into existing workflows is essential for achieving optimal results. By meticulously following these best practices, teams can ensure that the tools chosen not only meet immediate project needs but also support future growth and innovation.
Key insights highlighted throughout the article include:
Furthermore, the integration process should be handled with care, emphasizing training and feedback to facilitate a smooth transition and maximize productivity.
Ultimately, the choice of embedded development tools can either propel a project forward or hinder its progress. By adhering to these best practices, organizations can enhance their development processes, foster innovation, and ensure compliance with industry standards. Embracing these strategies not only contributes to immediate project success but also lays a foundation for future advancements in the rapidly evolving field of embedded systems.
Why is it important to define project requirements and constraints in embedded development for healthcare instruments?
Clearly outlining project requirements and limitations is essential to ensure the selection of appropriate embedded development tools, which helps mitigate risks and prevent complications during the production phase.
What types of requirements should be considered when developing healthcare instruments?
Both functional and non-functional requirements should be considered, including performance metrics, power consumption, and adherence to regulatory standards.
What are some examples of healthcare tools developed by Voler Systems?
Voler Systems has developed various healthcare tools, including wearable solutions, heart pumps, and liquid biopsy platforms.
How does Voler Systems enhance product development in healthcare instruments?
Voler Systems leverages AI-assisted engineering to accelerate product development and improve battery life through innovative power management strategies.
Why is stakeholder engagement important in the requirements gathering phase?
Engaging stakeholders early enriches the requirements gathering phase and provides critical insights that can significantly influence the project's trajectory.
What role do regulatory compliance and performance limitations play in embedded development?
Recognizing project constraints such as regulatory compliance and performance limitations is crucial for the effective use of embedded development tools in system design.
