4 Best Practices for Effective PCB Design Board in Medical Devices
Discover best practices for effective PCB design board in medical devices to ensure...

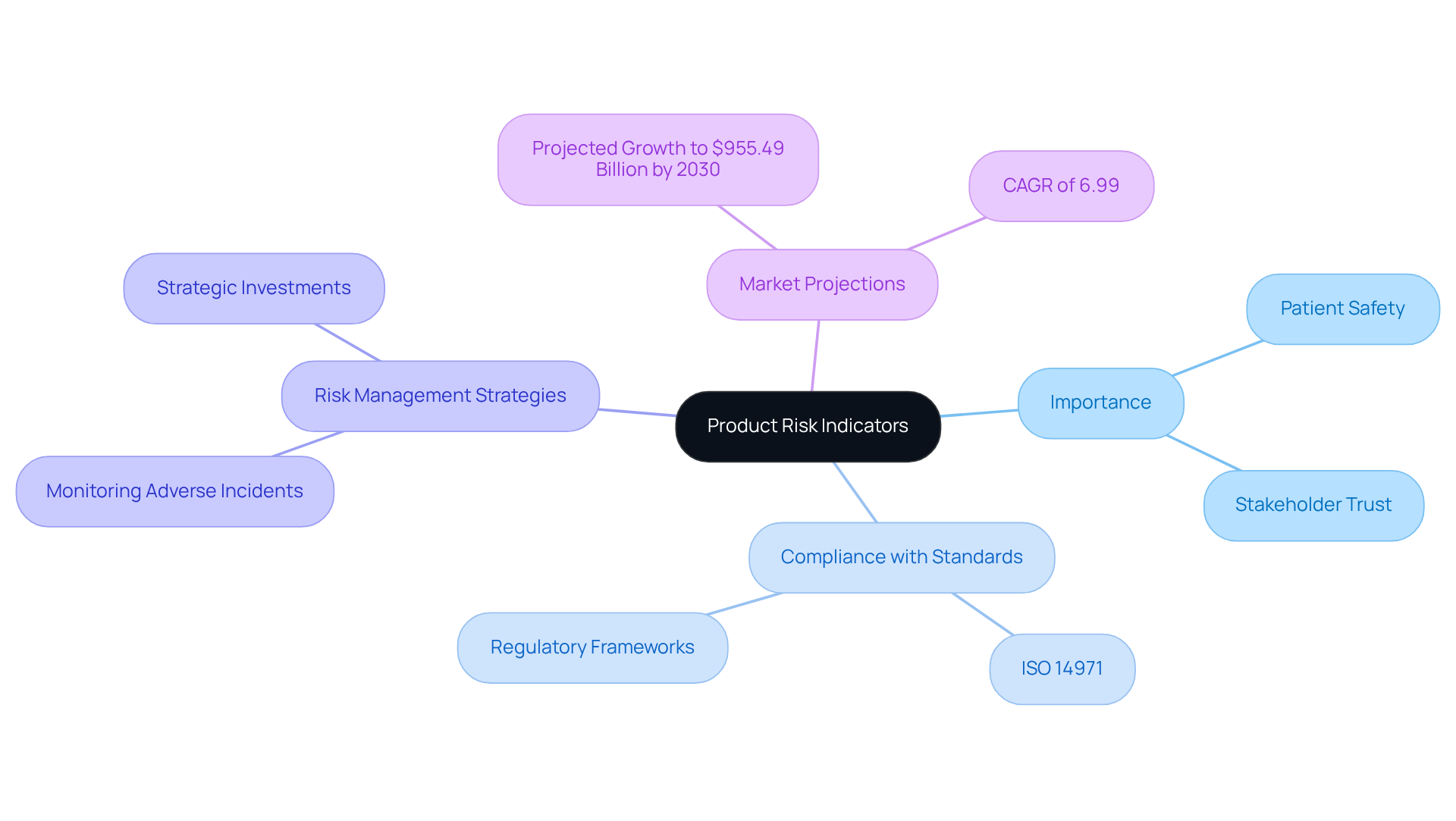
Product risk indicators play a crucial role in ensuring patient safety and compliance within the medical device industry. By effectively identifying and managing potential threats throughout a device's lifecycle, manufacturers can significantly enhance product reliability and foster stakeholder trust. However, given the complexity of evolving regulations and the increasing prevalence of risks, organizations must establish and maintain robust monitoring processes. These processes should not only comply with established standards but also contribute to improved patient outcomes.
Product risk indicators serve as crucial metrics for identifying, evaluating, and tracking potential threats associated with medical devices throughout their lifecycle. These indicators are essential for ensuring compliance with protection and efficacy standards mandated by regulatory frameworks such as ISO 14971.
By implementing strategic investments, manufacturers can effectively manage uncertainties, gaining insights into potential hazards that may compromise patient safety. For example, monitoring the frequency of adverse incidents reported for a specific device can help manufacturers identify trends and take timely actions to mitigate risks.
Notably, nearly one-third of healthcare IoT systems have reported critical risks, underscoring the importance of robust risk management strategies. Establishing well-defined procedures not only enhances compliance with regulatory standards but also fosters trust among stakeholders, ultimately contributing to safer medical products and improved patient outcomes.
As the medical equipment market is projected to reach $955.49 billion by 2030, the importance of effective product risk indicators cannot be overstated, particularly in light of the evolving landscape of equipment standards for effectiveness and reliability.
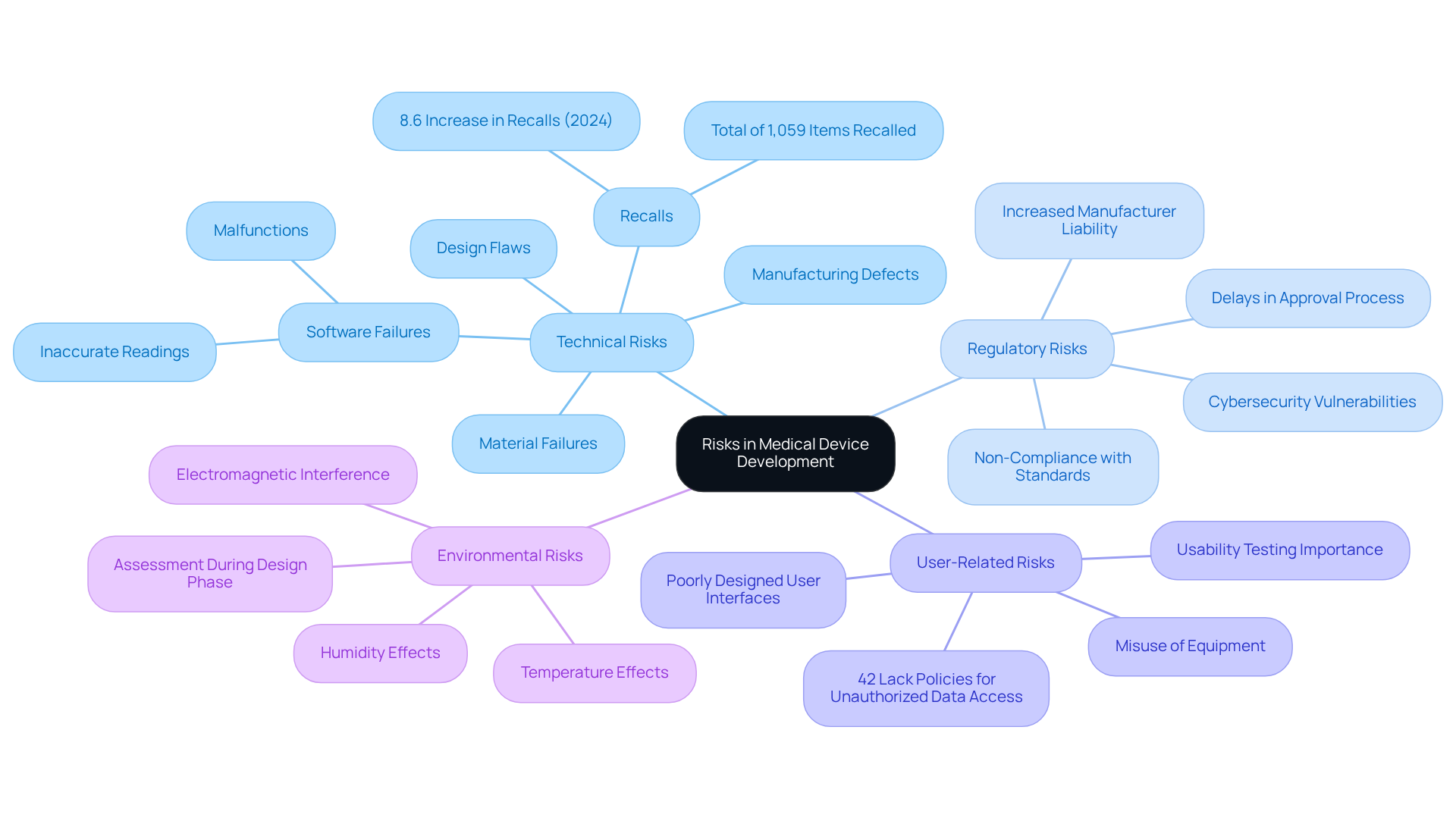
Recognizing pertinent dangers in medical equipment development requires a methodical strategy to identify potential threats that could compromise safety and functionality. Key areas to consider include:
Technical Risks: These encompass design flaws, material failures, and manufacturing defects. For example, software failures that manage a device can lead to inaccurate readings or malfunctions, contributing to an increase in medical product recalls, which rose by 8.6% year-over-year in 2024, totaling 1,059 items recalled.
Regulatory Risks: Non-compliance with regulatory standards can result in significant delays or outright rejections during the approval process. Understanding the specifications of ISO 14971 and the latest FDA guidelines is essential, especially as the new framework has heightened manufacturer liability and vulnerability regarding cybersecurity.
User-Related Risks: These stem from user interactions with the equipment. A poorly designed user interface can lead to misuse, potentially endangering patients. Conducting usability testing is vital to identify these issues early, as evidenced by the fact that 42% of healthcare organizations lack policies to prevent unauthorized data access, which can exacerbate user-related challenges.
Environmental Risks: Factors such as temperature, humidity, and electromagnetic interference can significantly impact device performance. Assessing these conditions during the design phase is crucial to mitigate hazards associated with environmental factors.
Employing methods like Failure Mode and Effects Analysis (FMEA) and conducting thorough literature reviews enables manufacturers to effectively identify and prioritize threats, ensuring a comprehensive management strategy that aligns with industry standards and enhances patient safety.
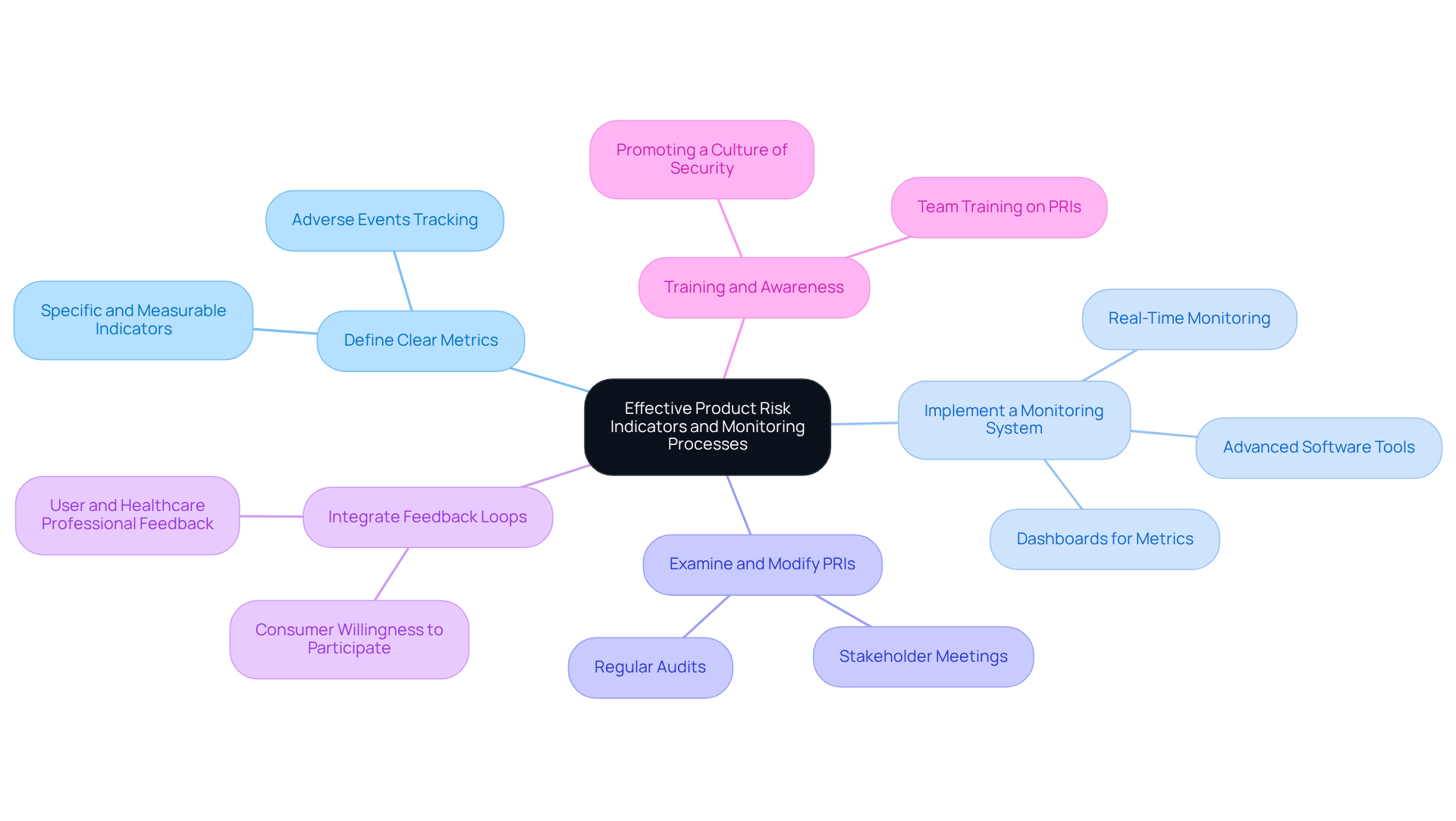
To establish effective product risk indicators (PRIs) and monitoring processes, manufacturers should adhere to the following best practices:
Define Clear Metrics: Develop specific, measurable, and relevant indicators that align with identified risks. For instance, tracking the rate of adverse events per device sold can provide insights into safety performance, which is crucial for maintaining compliance and enhancing patient safety.
Implement a Monitoring System: Utilize advanced software tools to automate data collection and analysis, ensuring real-time monitoring of performance-related indicators. Dashboards that display essential metrics facilitate swift evaluations, enabling manufacturers to react promptly to emerging challenges. For example, the University of Pittsburgh Medical Center reported a 76% reduction in hospital readmissions due to effective RPM systems, underscoring the importance of continuous monitoring.
Consistently Examine and Modify the Product Risk Indicator: As new challenges arise or existing ones evolve, it is crucial to assess and adapt the product risk indicator accordingly. Conducting regular audits and stakeholder meetings fosters a proactive approach to managing uncertainties, ensuring that the organization remains compliant with evolving regulatory standards.
Integrate Feedback Loops: Establish mechanisms for collecting feedback from users and healthcare professionals. Insights obtained from these stakeholders can uncover potential threats that may not be identified through conventional monitoring techniques. A survey indicated that between 65% and 70% of consumers are willing to participate in RPM programs, emphasizing the value of user feedback in identifying risks.
Training and Awareness: Ensure that all team members understand the importance of PRIs and are trained in how to monitor and report on them effectively. This promotes a culture of security and adherence within the organization, which is essential for satisfying regulatory expectations and improving overall product quality.
By adopting these practices, manufacturers can establish a robust management framework that not only meets regulatory requirements but also significantly enhances patient safety and satisfaction.
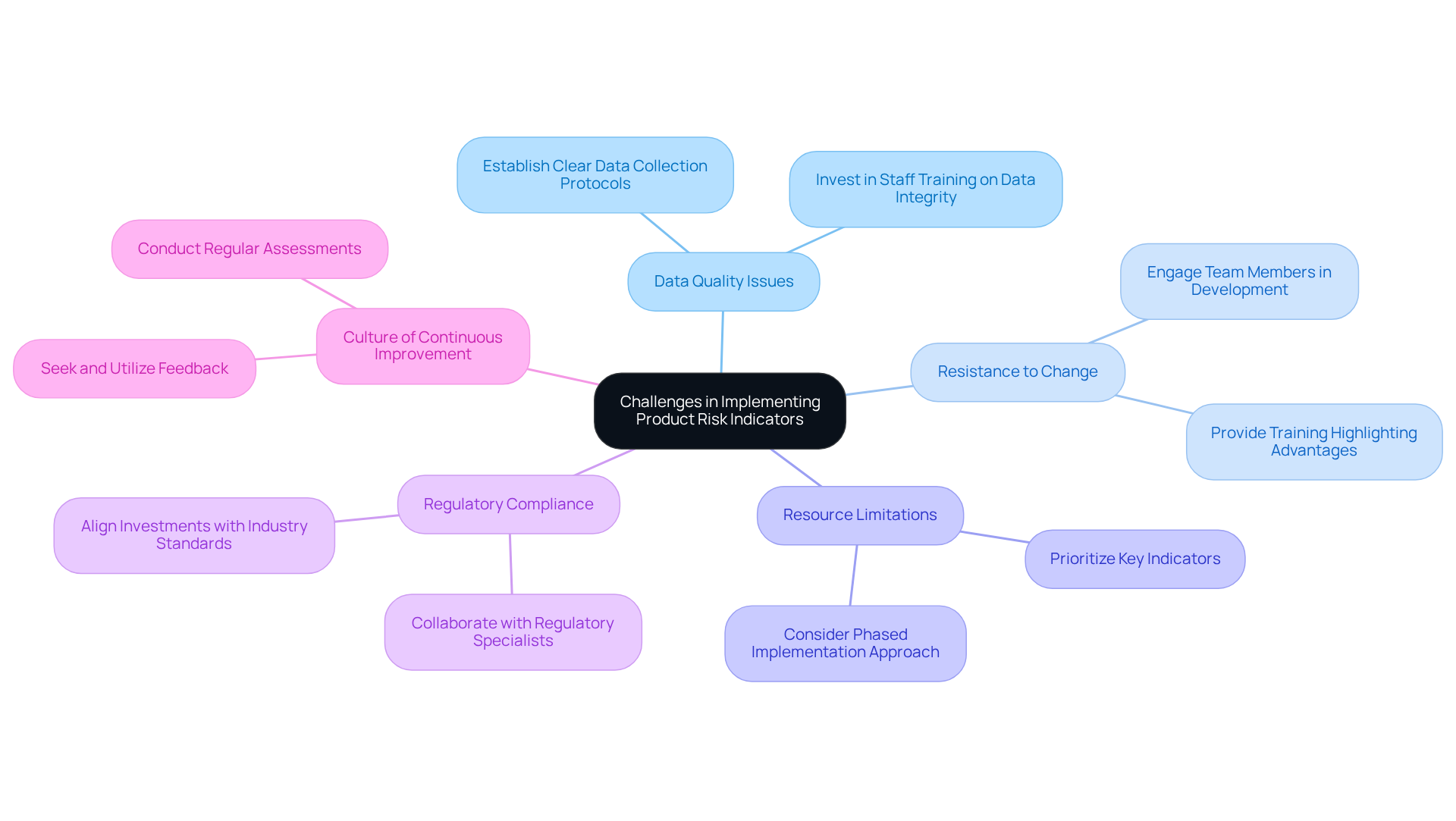
Implementing product risk indicators poses several challenges, which can be effectively addressed through strategic approaches:
By proactively addressing these challenges, organizations can enhance their risk management processes, ensuring that they effectively implement and maintain product risk indicators.
Effective product risk indicators (PRIs) are crucial for ensuring the safety and efficacy of medical devices throughout their lifecycle. By clearly defining these indicators and implementing strategic monitoring processes, manufacturers can proactively manage potential risks, comply with regulatory standards, and ultimately enhance patient safety. The significance of PRIs is paramount, particularly in a rapidly evolving industry where the stakes are high and the consequences of oversight can be severe.
This article highlights several key practices for establishing effective PRIs, including:
It also addresses the challenges manufacturers encounter, such as data quality issues and resistance to change, while offering solutions to overcome these barriers. By prioritizing these practices and fostering a culture of continuous improvement, organizations can significantly enhance their risk management frameworks.
In conclusion, the implementation of robust product risk indicators transcends mere regulatory compliance; it is a fundamental aspect of delivering safe and effective medical devices. By investing in effective risk management strategies, manufacturers can safeguard patient safety, build trust with stakeholders, and ultimately contribute to the advancement of healthcare. Embracing these best practices will pave the way for more reliable medical products and a safer healthcare environment.
What are product risk indicators in the context of medical devices?
Product risk indicators are metrics used to identify, evaluate, and track potential threats associated with medical devices throughout their lifecycle.
Why are product risk indicators important for medical devices?
They are essential for ensuring compliance with protection and efficacy standards set by regulatory frameworks, such as ISO 14971, and help manage uncertainties that could compromise patient safety.
How can manufacturers use product risk indicators to improve patient safety?
By monitoring indicators such as the frequency of adverse incidents reported for a device, manufacturers can identify trends and take timely actions to mitigate risks, thus enhancing patient safety.
What is the significance of risk management strategies in healthcare IoT systems?
Nearly one-third of healthcare IoT systems have reported critical risks, highlighting the need for robust risk management strategies to ensure safety and compliance.
How do well-defined procedures for product risk indicators benefit manufacturers?
Establishing clear procedures enhances compliance with regulatory standards, fosters trust among stakeholders, and contributes to the development of safer medical products and improved patient outcomes.
What is the projected market value of medical equipment by 2030?
The medical equipment market is projected to reach $955.49 billion by 2030, emphasizing the importance of effective product risk indicators in this evolving landscape.
