Understanding the Importance of Transmission Lines in PCB Design
Discover the significance of transmission lines in PCB design for optimal signal...
Ensuring the reliability of medical devices relies heavily on the often-overlooked aspect of signal integrity. This critical factor directly impacts both patient safety and device performance. As healthcare technology continues to advance, the necessity for robust signal integrity verification practices becomes increasingly important. Such practices provide engineers with a pathway to enhance the quality of data transmission in devices, which range from wearable monitors to complex diagnostic tools.
However, manufacturers face numerous challenges, including electromagnetic interference and crosstalk, which threaten signal quality. How can they effectively navigate these pitfalls to ensure compliance and safeguard patient outcomes?
Signal integrity verification is a crucial assessment of the quality of electrical transmission as it traverses a circuit, and its significance in medical equipment cannot be overstated. Even minor fluctuations in quality can lead to inaccurate readings or equipment failures, posing serious risks to patient safety. High-quality inputs are vital for accurate data transmission, especially in essential applications such as wearable health monitors and diagnostic devices, where signal integrity verification is crucial.
The FDA recognizes the importance of quality assurance, enforcing stringent testing criteria to ensure healthcare products meet rigorous standards. This regulatory framework underscores the necessity of incorporating robust communication quality measures during the design phase, as they are fundamental to the reliability and efficiency of healthcare equipment, particularly through signal integrity verification.
Recent updates to FDA regulations emphasize user comprehension and the need for clear explanations of algorithms used in clinical decision support software, reflecting a broader commitment to maintaining data quality throughout the equipment lifecycle. As healthcare technology evolves, the focus on signal integrity verification remains a pivotal factor in safeguarding patient well-being and enhancing the functionality of healthcare instruments.
Manufacturers must be cognizant of common pitfalls, such as overlooking the impact of distortion on patient safety or failing to adhere to FDA standards, to ensure the successful development of their products.
At Voler Systems, our expert electronic design services leverage AI-assisted engineering to enhance communication quality and battery life in wireless healthcare tools through signal integrity verification, ensuring they meet the highest performance and reliability standards. Our expertise spans the creation of various healthcare products, from wearable health monitors to advanced diagnostic instruments, all designed with a commitment to preserving communication quality and compliance with regulatory standards.
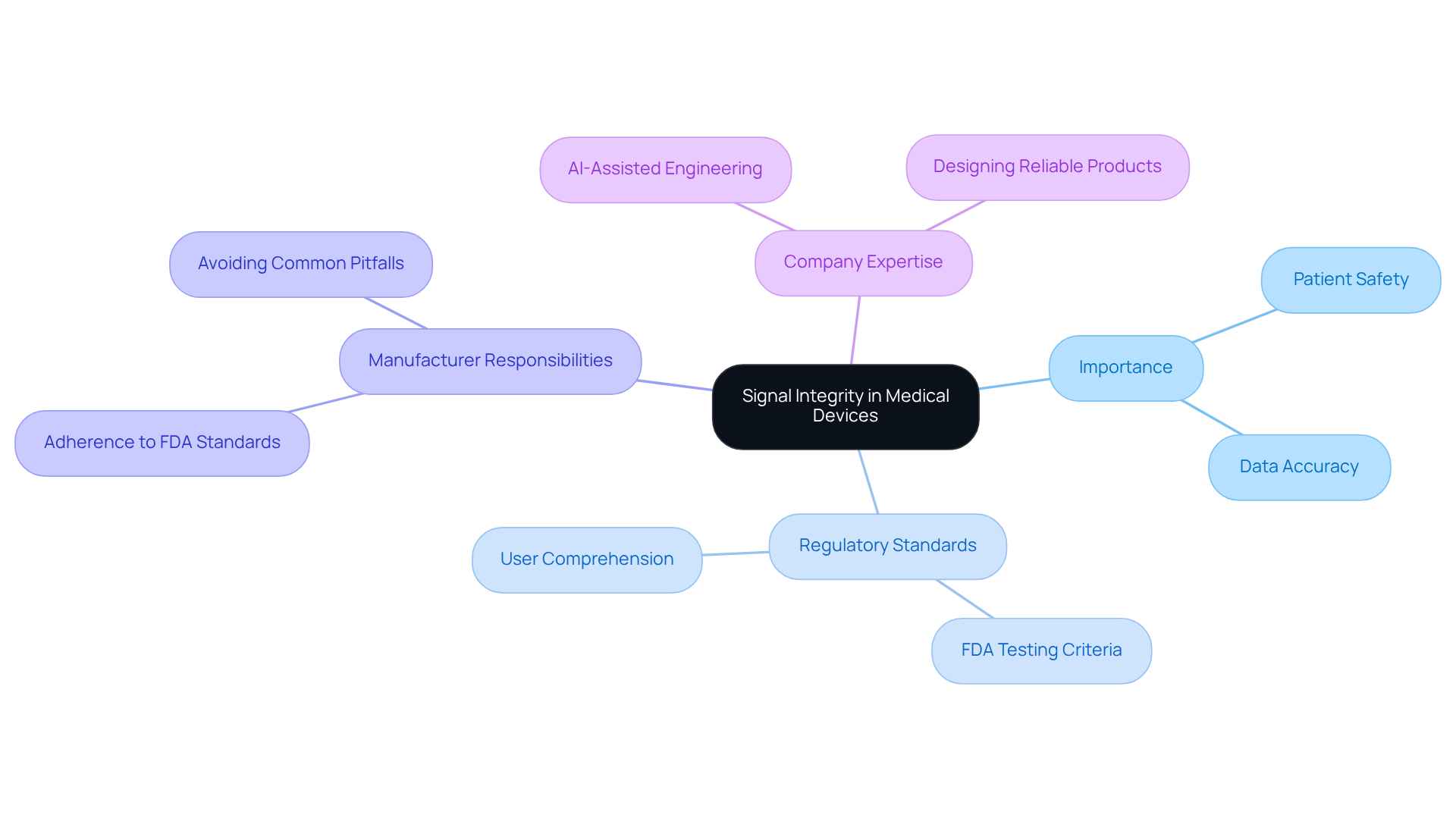
Signal quality in medical equipment is often compromised by issues such as crosstalk, attenuation, and electromagnetic interference (EMI). Crosstalk arises when signals from adjacent traces interfere with one another, leading to potential data corruption and unreliable device performance. This concern is particularly significant in densely populated PCBs, where multiple transmissions coexist. Attenuation, defined as the reduction of signal strength over distance, can be worsened by suboptimal PCB layouts, making careful consideration of trace routing and spacing essential.
EMI poses a substantial risk, especially in environments saturated with electronic devices, such as hospitals. Powerful sources of EMI, including electrosurgical instruments and MRI machines, can disrupt signal transmission, complicating the reliability of healthcare equipment. The transition to portable healthcare devices, like handheld ultrasound machines, has further complicated the anticipation of EMI sources, as these instruments operate in dynamic healthcare settings. As noted by Don Witters, Chairman of the electromagnetic compatibility (EMC) working group at the Center for Devices and Radiological Health (CDRH), "Because of the nature of radio transmissions, the closer you get to the transmitter antenna, the greater the field strength."
To address these challenges, engineers must adopt robust design strategies. Effective grounding techniques and multi-layer shielding solutions, such as conductive polymers and hybrid materials, are vital for safeguarding sensitive components from external noise. Additionally, optimizing trace routing and employing filtering methods can significantly enhance transmission quality. Voler Systems, leveraging its extensive experience in supporting legacy test equipment for healthcare technology firms, emphasizes the importance of signal integrity verification to address these challenges and ensure compliance with regulatory standards while maintaining product performance. Furthermore, common errors in manufacturing tests, such as inadequate grounding or improper trace routing, can lead to significant reliability issues. The global EMI shielding market is projected to reach $8.6 billion by 2027, underscoring the importance of these considerations in healthcare device design. Ensuring compliance while maintaining equipment efficacy is crucial for patient safety. As Charles Swanson, Vice President of pacing regulatory affairs and compliance at Medtronic, remarked, "When the issue of cellular phones and pacemakers came up... we started to do our in-house testing, and then worked with FDA to test our products.
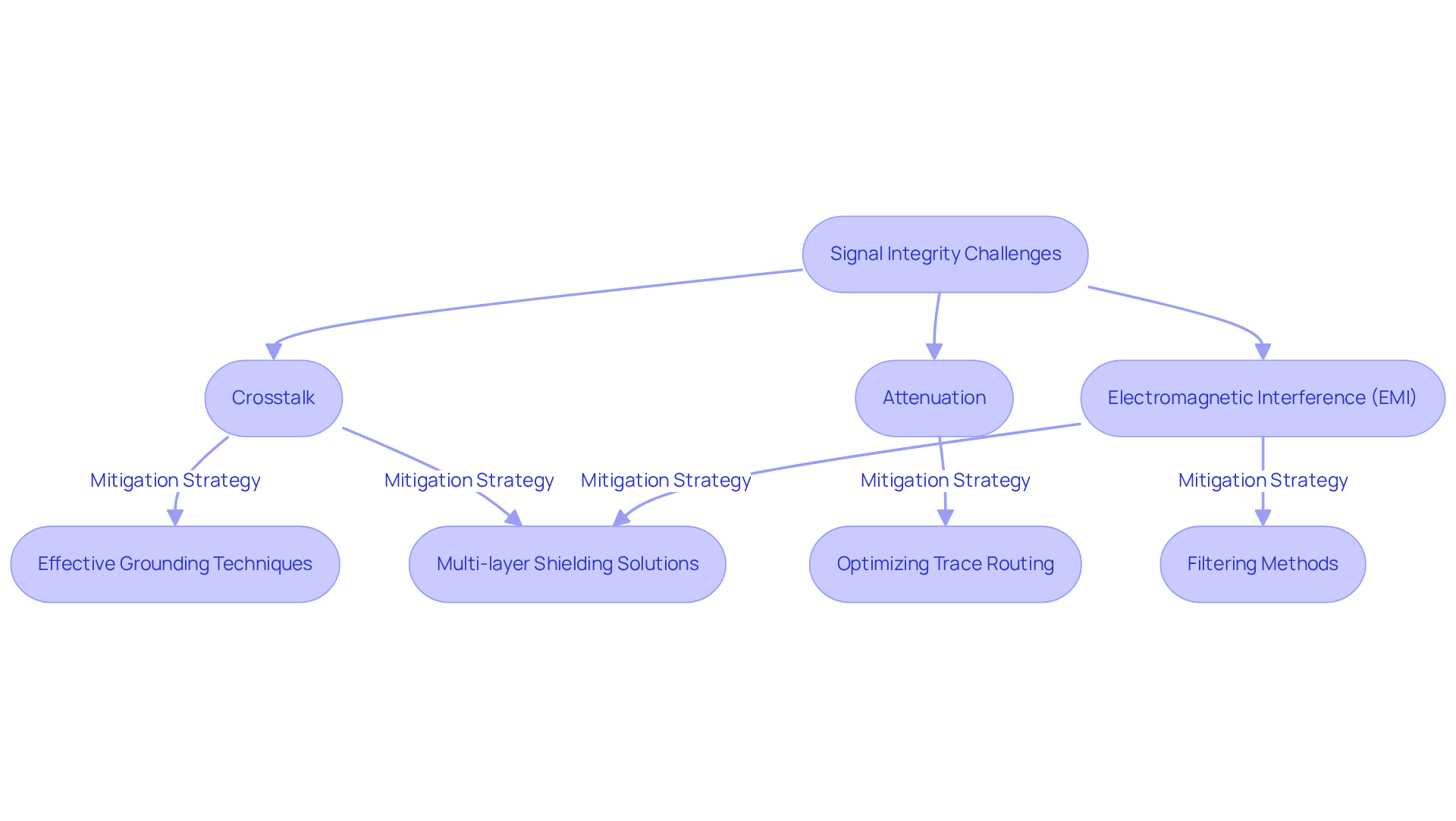
To ensure optimal signal integrity in medical devices, engineers should adopt several best practices:
Utilize Differential Signaling: This technique significantly reduces noise susceptibility, thereby enhancing the reliability of data transmission in high-speed applications. Differential signaling effectively eliminates common-mode noise, which is crucial for preserving quality in environments with electromagnetic interference (EMI).
Implement Proper Impedance Matching: Matching the impedance of traces minimizes reflections that can distort transmissions. This practice is particularly vital in high-speed designs, where even minor mismatches can lead to considerable performance degradation.
Optimize Trace Lengths and Widths: Keeping trace lengths short and widths appropriate reduces resistance and inductance, both essential for maintaining the integrity of the transmission. Techniques such as avoiding sharp corners and utilizing 45-degree angles can further enhance performance by minimizing reflections.
Utilize Ground Planes: A solid ground plane provides a stable reference for communications and helps minimize EMI. This is particularly important in healthcare instruments, where noise can disrupt sensitive measurements. Strategic placement of vias can connect components to the ground plane, ensuring low-impedance paths for return currents.
Conduct Thorough Testing: Implementing rigorous testing at various stages of development enables engineers to identify and rectify issues early. Testing multiple units under worst-case conditions can instill confidence that at least one unit meets specifications, thereby ensuring compliance with industry standards.
By adhering to these optimal methods, engineers can enhance transmission quality through signal integrity verification, contributing to the overall dependability and efficiency of healthcare instruments.
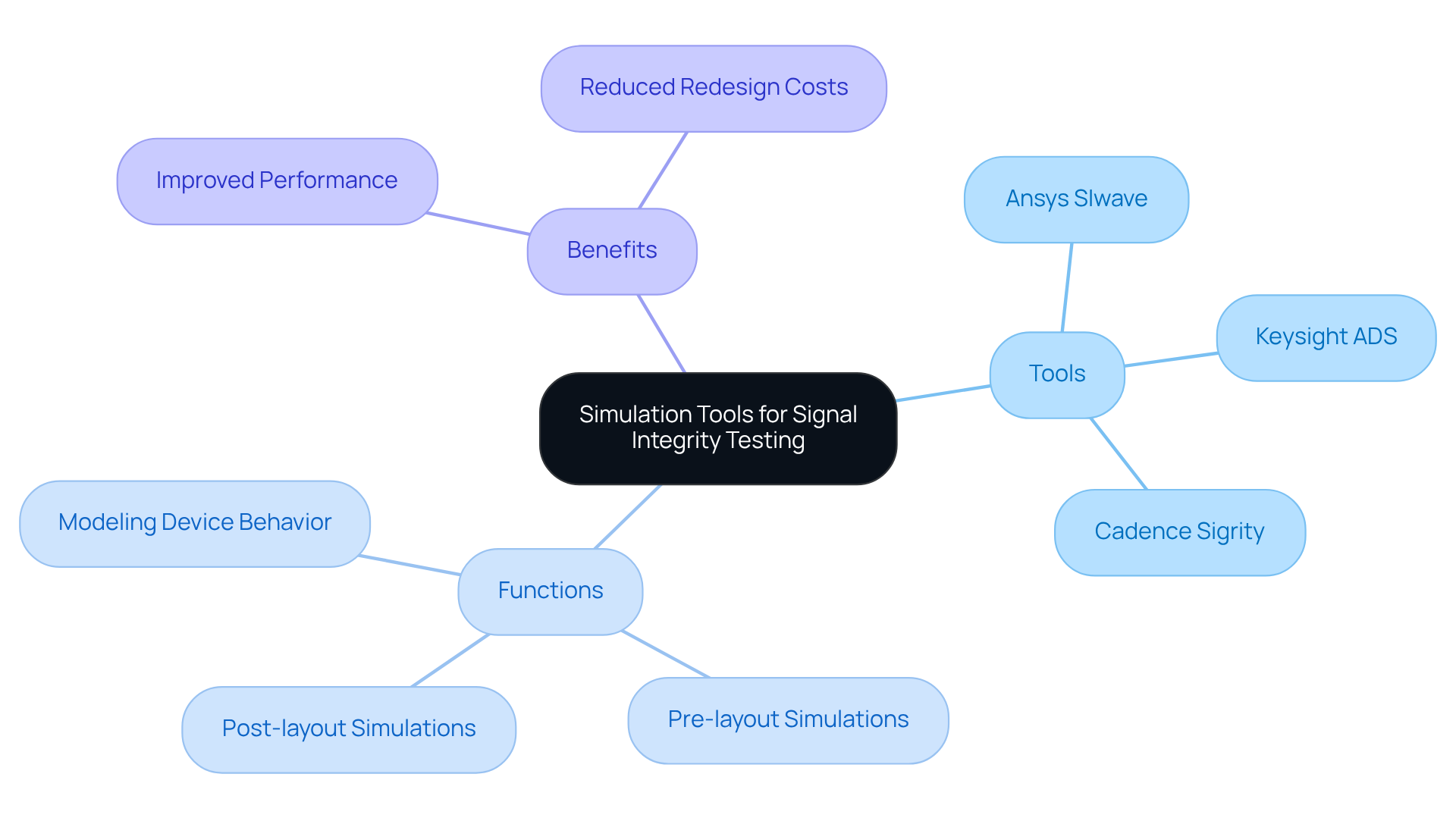
Simulation tools such as Ansys SIwave, Keysight ADS, and Cadence Sigrity play a vital role in assessing data quality within medical devices. These sophisticated tools enable engineers to model device behavior under diverse conditions, effectively pinpointing potential issues like crosstalk and degradation before physical prototypes are developed.
By conducting both pre-layout and post-layout simulations, engineers can refine designs, ensuring that signal integrity is maintained throughout the development process. This proactive approach not only improves performance but also significantly mitigates the risk of costly redesigns.
Notably, recent updates to Ansys SIwave and Cadence Sigrity in 2026 have further augmented their capabilities, solidifying their importance in healthcare product design. For instance, case studies demonstrate how Keysight ADS has successfully addressed quality challenges in healthcare projects, underscoring the effectiveness of these simulation tools in delivering reliable and high-performance health solutions.
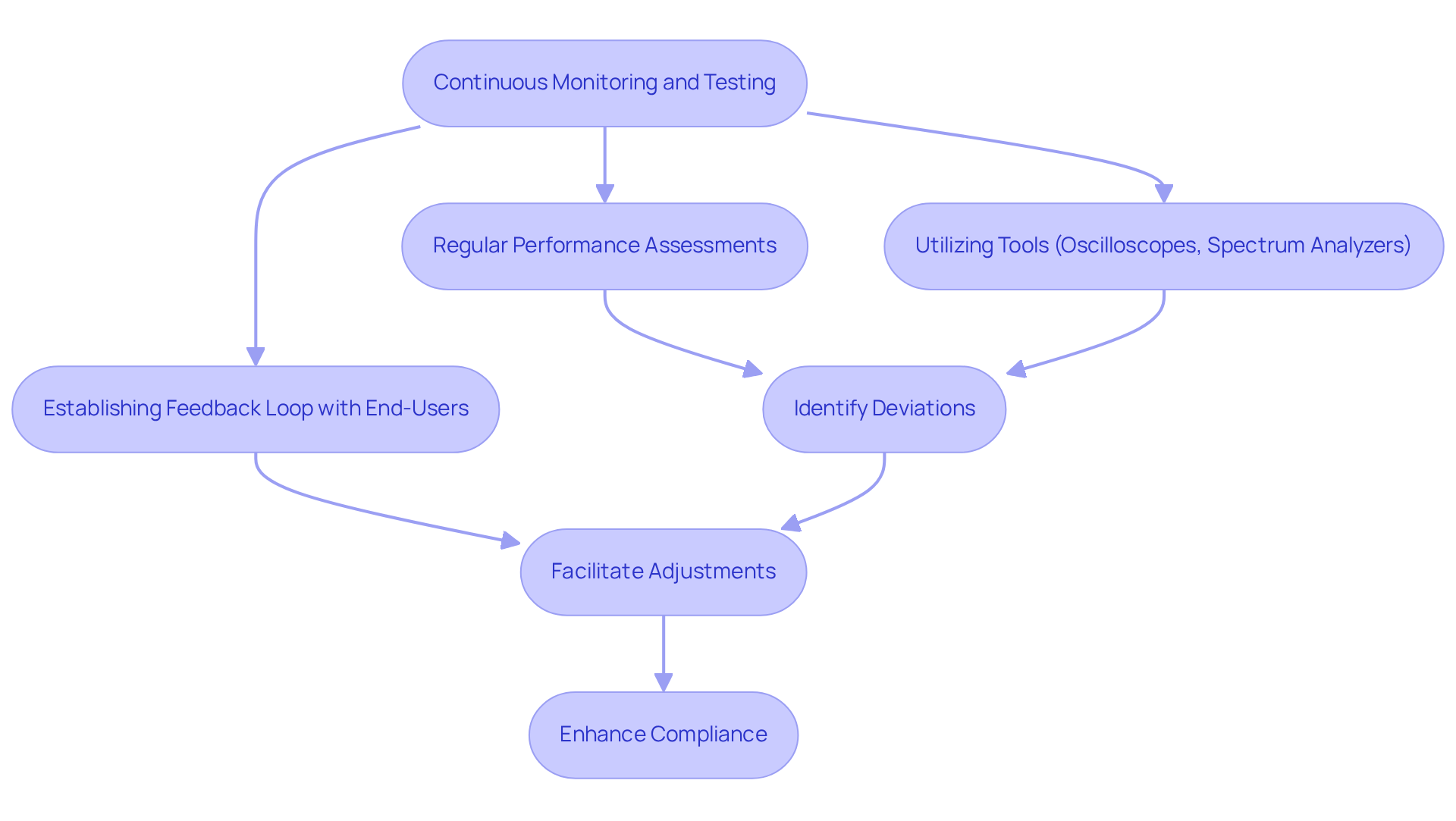
To ensure the integrity of communications throughout the lifecycle of medical equipment, continuous monitoring and testing protocols are essential. Regular performance assessments, utilizing tools such as oscilloscopes and spectrum analyzers, are crucial for signal integrity verification in identifying deviations in signal quality. Establishing a feedback loop with end-users provides valuable insights into real-world performance, facilitating timely adjustments and enhancements. This proactive strategy helps uphold compliance with safety and effectiveness standards while addressing complexities related to performance in dynamic healthcare environments.
Frequent errors in establishing manufacturing tests - such as insufficient calibration, incorrect testing conditions, and inadequate data analysis - can lead to hardware failures, a significant cause of post-market incidents, particularly in orthopedic implants and cardiac instruments. This highlights the necessity for rigorous testing and monitoring. Furthermore, the FDA has sought public feedback on measuring and evaluating the performance of AI-enabled healthcare tools, underscoring the importance of continuous assessments in the current regulatory landscape.
By prioritizing ongoing signal integrity verification and leveraging expert development of wearable sensors, manufacturers can significantly enhance the reliability and efficacy of their medical devices, ultimately improving patient outcomes. As Andrea Downing noted, from the patient perspective, approval is not the finish line; it is the starting line, emphasizing the need for continuous performance monitoring post-deployment.
Ensuring signal integrity in medical devices is crucial for safeguarding patient safety and enhancing the reliability of healthcare technologies. Even minor disruptions in signal quality can lead to significant risks, making it essential for manufacturers to adopt rigorous verification practices throughout the design and development process.
Key insights highlight common challenges such as:
Along with best practices for addressing these issues. Techniques like:
Are vital for maintaining high standards of signal integrity. Furthermore, leveraging advanced simulation tools enables engineers to preemptively identify potential problems, ensuring that devices function reliably in real-world scenarios.
Given the critical role that signal integrity plays in the performance and safety of medical devices, a proactive approach to verification and ongoing testing is not merely advisable but necessary. Manufacturers are urged to prioritize these practices to enhance device efficacy and ultimately improve patient outcomes, recognizing that the journey toward excellence in healthcare technology is continuous.
What is signal integrity and why is it important in medical devices?
Signal integrity refers to the quality of electrical transmission as it moves through a circuit. It is crucial in medical devices because even minor fluctuations can lead to inaccurate readings or equipment failures, posing serious risks to patient safety. High-quality inputs are essential for accurate data transmission, particularly in wearable health monitors and diagnostic devices.
How does the FDA regulate signal integrity in medical devices?
The FDA enforces stringent testing criteria to ensure that healthcare products meet rigorous standards, highlighting the importance of quality assurance. This regulatory framework necessitates robust communication quality measures during the design phase, which are fundamental to the reliability and efficiency of healthcare equipment.
What recent updates have been made to FDA regulations regarding signal integrity?
Recent updates emphasize user comprehension and the need for clear explanations of algorithms used in clinical decision support software. This reflects a broader commitment to maintaining data quality throughout the equipment lifecycle.
What common challenges affect signal integrity in medical device design?
Common challenges include crosstalk, attenuation, and electromagnetic interference (EMI). Crosstalk occurs when signals from adjacent traces interfere with each other, leading to potential data corruption. Attenuation is the reduction of signal strength over distance, which can be exacerbated by poor PCB layouts. EMI poses risks in environments with many electronic devices, such as hospitals.
How can engineers address signal integrity challenges in medical devices?
Engineers can adopt robust design strategies, including effective grounding techniques and multi-layer shielding solutions, to protect sensitive components from external noise. Additionally, optimizing trace routing and employing filtering methods can significantly enhance transmission quality.
What are the implications of failing to address signal integrity issues in medical devices?
Failing to address signal integrity issues can lead to unreliable device performance and significant reliability problems, which can compromise patient safety. Common manufacturing errors, such as inadequate grounding or improper trace routing, can exacerbate these issues.
What is the projected growth of the global EMI shielding market and its relevance to healthcare device design?
The global EMI shielding market is projected to reach $8.6 billion by 2027, underscoring the importance of addressing EMI concerns in healthcare device design to ensure compliance and maintain equipment efficacy for patient safety.
