Company: Fortune 500 Medical Device Company
Industry: Medical Devices
Use Case: Class III Heart Pump Console
Services Used: Software, Diagnostics, Documentation, Verification
Results:
- Zero comments or conditions from the FDA on software for Class III medical devices.
- Following FDA approval with no software revision mandates, the heart pump went to clinical trials more quickly.
- Systemic verification and traceability analysis ensured compliance with regulations and minimized patient risks.
Customer Introduction
This large medical device company sells cardiac equipment. It acquired a smaller company with a proof-of-concept heart pump that serves as a lifesaving solution for people undergoing heart surgery.
Problem/Goal
- The organization lacked software expertise to develop software for a Class III medical device.
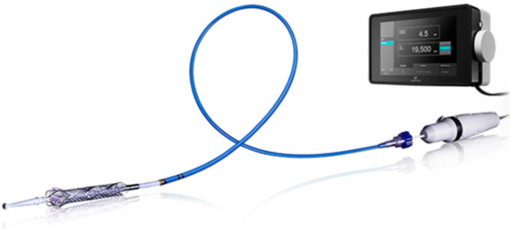
The pump's design augments the heart's pumping function during surgery and reduces the risk of death for patients with a high mortality rate. The pump folds into a catheter, is inserted into the femoral vein in the thigh, guided to the heart, and then is unfolded to begin pumping. The heart pump includes a console with a display, sensors, and controls.
- Needed to modify the pump's electronics and provide the user with error alerts to prevent device failure.
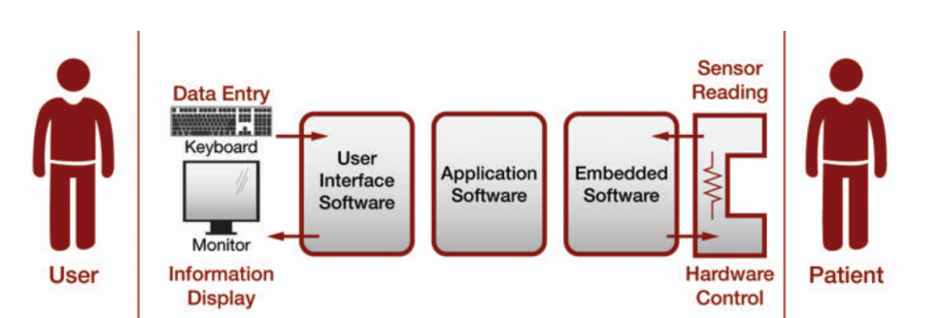
The console measures and controls the speed of the pump and, therefore, the blood volume. It also measures pressure and detects any blood leakage into the pump drive system. The software had to control the pump, measure sensors, and provide an intuitive user interface to alert the user of any potential problems.
Solution
This company engaged with Voler Systems because of its extensive experience in medical device design and development and strict federal regulatory requirements.
Voler Systems' engineers identified what was needed to take the pump from a proof-of-concept to reality. They implemented a development process to ready the heart pump for submission to the FDA. This systematic approach resulted in Voler Systems delivering the following solutions:
- Wrote all software for the console
- Modified electronics and created user-interface software, including more than 100 error messages, to prevent operator error
- Created and executed the verification protocol
- Created document package for software and verification results in the quality system
Results & Benefits
Upon review of the system, the FDA approved the software without any conditions or comments. This is significant, as there is a rare occurrence of no software modifications required by the FDA in a Class III medical device.
Voler System's approach helped ensure compliance with rigorous FDA regulatory requirements, provided confidence in the heart pump's safety and efficacy, and allowed the device to move to clinical trials more quickly.
Inspiration
Using a systematic approach, coupled with a passion for innovation and quality, Voler Systems can offer you the expertise needed to develop a safe and reliable medical device that meets FDA standards and clinical needs.
"Voler Systems' expertise and attention to detail were instrumental in developing a heart pump console that met regulatory standards, exceeded clinical needs, and ensured patient safety."
Contact us and let us help you achieve success as you make a positive impact on patient care.
